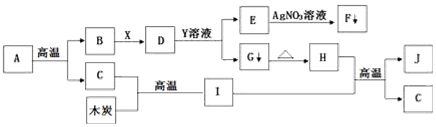
��Ŀ����
3����ѧ������������أ���1��ͼ1ΪijʳƷ��װ���IJ���˵������ش��������⣺
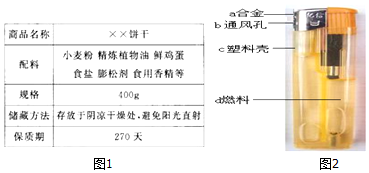
���ڱ��ɵ������У�������֬���Ǿ���ֲ���ͣ�
�ڸ������и��������ʵ��������ʼ�����
��С����и������࣬�����������ھ���һϵ�еı仯����ת��Ϊˮ��CO2���ѧʽ����
�ܴ�Ӫ������ĽǶȿ�����ʳƷ�г�ˮ�⣬��ȱ�ٵ�Ӫ������ά���أ�
��2��ͼ2�Ǵ�����ʾ��ͼ��������й��� Ϣ�ش��������⣺
�ٹ��ɴ����IJ��������ںϳɲ��ϵ���c�����ţ���
����ȼ�ϵ���Ҫ�ɷֶ��飨C4H10�������л�����л�����������������
�۴���������ͨ���������ʹ������ͨ��˵����ȼ��ȼ�ձ����������Ӵ�����ͨ���ͨ��������������ɴ���������ȼ�ϲ���ȫȼ�ն������ж�����CO���ѧʽ����
���� ��1���ٸ���ʳƷ�и�����Ӫ���ؽ��н��
�ڸ���ʳƷ�и�����Ӫ�����Լ�������������Ӫ������������
�۸���С�����ۣ������������ھ���һϵ�еı仯����ת��Ϊˮ�Ͷ�����̼���з�����
�ܸ���ʳƷ�и�����Ӫ�����Լ�������������Ӫ������������
��2���ٸ��ݲ��ϵķ�����з�����
�ڸ����л�������ĸ�����з�����
�۸���ȼ����Ҫͬʱ���������������ٿ�ȼ�����������������¶�Ҫ�ﵽ�Ż�㣬��̼Ԫ�ص�ȼ��ȼ�ղ���ֻ�����һ����̼�����з������
��� �⣺��1�����ڱ��ɵ������У�������֬���Ǿ���ֲ���ͣ�
�������ֲ���ͣ�
�ڸ������и��������ʵ��������ʼ�����
����ʼ�����
��С����к��е�����������Ҫ�ǵ��ۣ������������ھ���һϵ�еı仯����ת��Ϊˮ�Ͷ�����̼��
���CO2��
��������Ҫ������Ӫ�����ʣ������ʡ����ࡢ��֬��ά���ء����κ�ˮ����Ӫ������ĽǶȿ�����ʳƷ�г�ˮ�⣬��ȱ�ٵ�Ӫ������ά���أ�
���ά���أ�
��2�����������ںϳɲ��ϣ�
�ڶ��麬��̼Ԫ�أ������л������
�۴���������ͨ���������ʹ������ͨ��˵��ȼ��ȼ����Ҫ�������Ӵ�����̼Ԫ�ص�ȼ��ȼ�ղ���ֻ�����һ����̼����ͨ���ͨ��������������ɴ���������ȼ�ϲ���ȫȼ�ն������ж���һ����̼�����˷���ȼ������Ⱦ�˻�����
�����c���������������CO��
���� ���⿼����ǻ�ѧ�������֪ʶ����ɴ��⣬�����������е�֪ʶ���У�

 ��У����ϵ�д�
��У����ϵ�д�| �� | �� | �� | �� | |
| ��Ӧǰ������/g | 4 | 10 | 1 | 25 |
| ��Ӧ��tʱ�̵�����/g | 2 | a | b | 17 |
| ��Ӧ�������/g | 0 | 22 | 9 | d |
| A�� | d����ֵΪ9 | B�� | b����ֵΪ4 | ||
| C�� | �Ͷ�Ϊ��Ӧ�� | D�� | ������Է�������Ϊ��2�� |
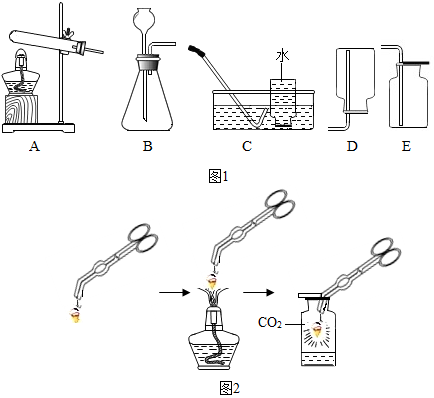
��1����ȡ����
����ѡ��A��C�����ȡ��������Ӧ�Ļ�ѧ����ʽΪ2KClO3$\frac{\underline{MnO_2}}{��}$2KCl+3O2����
����ʯ��ʯ��ϡ������ȡCO2�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2����ѡ�õķ������ռ�װ����BE�����ţ������Ӻ�װ�ã���ҩƷ����װ��ǰӦ���еIJ����Ǽ��װ�õ������ԣ�
��2��̽�����������
ijͬѧ�ڿ����е�ȼþ����������CO2��Ϩ��ȼ�յ�þ�����������þ���ڳ���CO2�ļ���ƿ��û��Ϩ������ȼ�գ�����ƿ�ڱڳ��ֺ�ɫ���壬ƿ�׳��ְ�ɫ���壬ʵ�������ͼ2��ʾ����ش�
�ټг�þ������������������ǯ��
�����ɵĺ�ɫ������C��д��ѧʽ����
�۲������ϣ�MgO��Mg��OH��2��MgCO3��Ϊ������ˮ�İ�ɫ���壮ͬѧ���ۺ���Ϊ��ɫ���岻������Mg��OH��2�������Ƿ�Ӧ����û����Ԫ�أ�
��Ϊ��һ��ȷ����ɫ����ijɷ֣���������ʵ�飺
| ʵ����� | ʵ������ | ʵ����� |
| ȡ��ɫ�������Թ��У���������ϡ���� | ��ɫ��������ʧ��û������ð�� | ��ɫ���岻��MgCO3������MgO |
| A�� | ͭ | B�� | ������̼ | C�� | ���ʯ | D�� | �������� |
| ����� | �������� | ��ȥ���ʵķ��� | |
| A | CO2���� | CO���� | ͨ����������ȼ |
| B | CuSO4��Һ | H2SO4��Һ | ��������Cu�ۣ���ַ�Ӧ����� |
| C | C�� | Fe�� | �����������ᣬ��ַ�Ӧ����� |
| D | KMnO4 | MnO2 | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
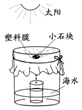 ˮ����Ҫ��Դ��
ˮ����Ҫ��Դ��