��Ŀ����
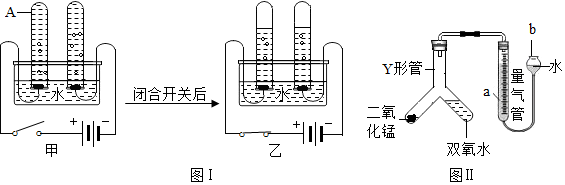
18��Ϊ�˲ⶨij̼������Ʒ��̼���Ƶ������������������ʲ�����ˮ��Ҳ�����������ʷ�Ӧ����ij��ѧС�����������ʵ�飺
��ش��������⣺
��1��д��������Ӧ�Ļ�ѧ����ʽNa2CO3+CaCl2�TCaCO3��+2NaCl��
��2�������Ȼ��Ƶ������г������Ʒ�д����������x������ʽ��$\frac{106}{111}$=$\frac{x}{11.1g}$
��3����Ʒ��̼���Ƶ���������Ϊ80%��
��4��������Ӧ�����Һ����������11.6gˮ�����������ò�������Һ�����ʵ���������Ϊ11.7%��
��5����������������Ʒ132.5t��ȡ�ռ����Ƶô���Ϊ80%���ռ�100t��
���� ���ڹ��˵õ��Ĺ��庬�����ʣ����Բ������ڷ���ʽ�ļ��㣮���ݼ�����Ȼ�����Һ�������Ͷ�Ӧ�������������㣮
��� �⣺
̼���ƺ��Ȼ��Ʒ�Ӧ�����Ȼ��ƺ�̼��Ƴ�������Ӧ�Ļ�ѧ����ʽΪNa2CO3+CaCl2�TCaCO3��+2NaCl��
��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy��̼��Ƶ�����Ϊz
�Ȼ��Ƶ�����Ϊ111g��10%=11.1g
Na2CO3+CaCl2�TCaCO3��+2NaCl
106 111 100 117
x 11.1g z y
$\frac{106}{x}$=$\frac{111}{11.1g}$=$\frac{100}{z}$=$\frac{117}{y}$
x=10.6g
y=11.7g
z=10g
��Ʒ��̼���Ƶ���������Ϊ$\frac{10.6g}{10.6g+12.65g-10g}$��100%=80%��
��4��������Ӧ�����Һ����������11.6gˮ�����������ò�������Һ�����ʵ���������Ϊ$\frac{11.7g}{10.6g+111g-10g-11.6g}$��100%=11.7%��
��5����������������Ʒ132.5t��ȡ�ռ����Ƶô���Ϊ80%���ռ� ������Ϊa��
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
106 80
132.5t��80% 80% a
$\frac{106}{80}$=$\frac{132.5t��80%}{80%a}$
a=100t
�ʴ𰸣�
��1��Na2CO3+CaCl2�TCaCO3��+2NaCl��
��2��$\frac{106}{111}$=$\frac{x}{11.1g}$
��3��80%��
��4��11.7%��
��5��100t��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 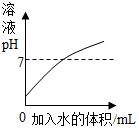 ��ϡ�����в��ϼ�ˮ | |
| B�� |  ��һ���������Ȼ�ͭ��ϡ����Ļ����Һ����μ�������������Һ | |
| C�� |  �ֱ��������������п�еμ�ϡ���� | |
| D�� | 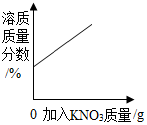 20��ʱ����һ�����Ľӽ����͵��������Һ�м�������ع��� |
| A�� | ������̼ͨ��ʯ����Һ | |
| B�� | ����̿���뵽���к�īˮ��ˮ�У��� | |
| C�� | ������̼ͨ���ռ���Һ | |
| D�� | ����ͭ���뵽ϡ�����У��� |
| A�� | �ƾ���Һ�� | B�� | �������� | C�� | ú�͡����� | D�� | ����ʯ��ʯ��ʯ |
| A�� | ���ױ�����ˮ�� | B�� | ��ʯ�ұ������ձ��� | ||
| C�� | Ũ�����ܷⱣ�����Լ�ƿ�� | D�� | ���ᱣ������ɫ�Լ�ƿ�� |