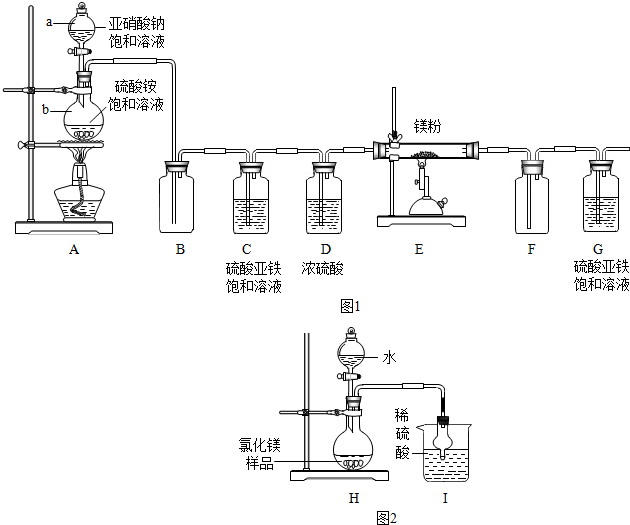
��Ŀ����
19��ij����С���һʪ��ұͭ���ŷŵķ�ˮ�к������������ͭ������Ⱦ�Ϊ�ⶨ�÷�ˮ�и���Ⱦ��ĺ�������ұͭ���ṩ������ˮ�IJο�������С���ͬѧ����������ʵ�飮ȡ��ˮ200g�������м���������������Ϊ20%������������Һ��������ɳ����������������������������Һ��������ϵ���±���| ���������������Һ������/g | 40 | 80 | 120 | 160 |
| ���ɳ���������/g | 0 | 9.8 | 19.6 | 19.6 |
��1����200g�÷�ˮ�м�������������������Һ��������������Ϊ19.6g��
��2��200g�÷�ˮ������ͭ����������������
��3��200g�÷�ˮ���������������Ϊ9.8g��
��4����ȡͬ����200g�÷�ˮ�������м���17.1%��Ba��OH��2��Һ�����㻭������Ba��OH��2��Һ����������������������Ĺ�ϵͼ�����ڴ������������ͼ������������۵�����꣩
���� ��1�����ݱ������ݷ�������������������
��2������������ͭ��������Ϸ���ʽ�������ͭ����������һ�������ˮ������ͭ����������������
��3���ɱ������ݿ�֪ÿ40g����������Һ������ͭ��Ӧ���ɳ���9.8g������ǰ40g����������Һ�����ᷴӦ�������������Ƶ��������������������
��4���ֱ����������Ba��OH��2��Һǡ�÷�Ӧ�ij�������������Ba��OH��2��Һ�����Լ�����ͭ��Ba��OH��2��Һǡ�÷�Ӧ�ij�������������Ba��OH��2��Һ������������ߣ�
��� �⣺��1�����ݱ������ݷ�����������������Ϊ19.6g
��2����200g��ˮ��CuSO4������Ϊx��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160 98
x 19.6g
$\frac{160}{x}$=$\frac{98}{19.6g}$
x=32g
��ˮ������ͭ��������������Ϊ$\frac{32g}{200g}$��100%=16%
�𣺷�ˮ������ͭ��������������Ϊ16%��
��3���ɱ������ݿ�֪ÿ40g����������Һ������ͭ��Ӧ���ɳ���9.8g������ǰ40g����������Һ�����ᷴӦ��
���ˮ�����������Ϊy
H2SO4+2NaOH=Na2SO4+2H2O
98 80
y 40g��20%
$\frac{98}{y}$=$\frac{80}{40g��20%}$
y=9.8g
�𣺷�ˮ�����������Ϊ9.8g
��4�����Һ��������Ba��OH��2��Һǡ�÷�Ӧ�����ᱵ����Ϊn������Ba��OH��2��Һ����m
H2SO4+Ba��OH��2=BaSO4��+H2O
98 171 233
9.8g m��17.1% n
$\frac{98}{9.8g}$=$\frac{171}{m��17.1%}$=$\frac{233}{m}$
m=100 n=23.3g
���Һ������ͭ��Ba��OH��2��Һǡ�÷�Ӧ�����ᱵ����Ϊa��������ͭ����Ϊb������Ba��OH��2��Һ����c
CuSO4+Ba��OH��2=BaSO4��+Cu��OH��2��
160 171 233 98
32g c��17.1% a b
$\frac{160}{32g}$=$\frac{171}{c��17.1%}$=$\frac{233}{a}$=$\frac{98}{b}$
a=46.6g b=19.6g c=200g
�������Һ�����������ͭ��Ӧ��ȫʱ�����ɳ���Ϊ23.3g+46.6g+19.6g=89.5g������Ba��OH��2��Һ����Ϊ100g+200g=300g
���Լ���Ba��OH��2��Һ����������������������Ĺ�ϵͼΪ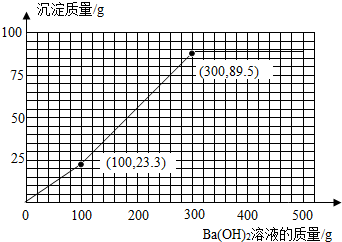
���꣨100��23.3���ͣ�300��89.5��
���� ������һ���ۺ��Ժ�ǿ�ĸ��ݻ�ѧ����ʽ���㣬ֻҪ����֪������Ӧδ֪���ǽ�������Ŀ�Ĺؼ����ڣ�������ͼҪ����ʼ����۵㼴�ɣ�

| A�� | θ�����Ļ��߿��Է��ú�Al��OH��3 | B�� | ��ɫʳƷ�����κλ�ѧ���� | ||
| C�� | �����ˡ��ܹ���Ӳˮת������ˮ | D�� | �ü�ȩ��Һ������ʳƷ�ı��ʼ� |
| A�� | ���亽��ɻ� | B�� | ˮ������ | C�� | ̫���ܹ��� | D�� | �������� |
| A�� | 45% | B�� | 50% | C�� | 56% | D�� | 60% |
| A�� | ���� | B�� | ���� | C�� | ���� | D�� | ������̼ |