��Ŀ����
��ͭΪͭп�Ͻ���;��Ϊ�㷺��С��ͬѧ�Ӽ�������˻�ͭ��Ƭ������ͬѧ��һ�����������е�ͭ���������Ǿ����Ȳⶨ��ͭ���ᷴӦ�����������������ټ��㣮
��С������Ƴ�����ͼA�����ⶨ��
������ͭ��Ʒ����ƿ��ϡ�����������
��ͭ��Ʒ������ƿ������ϡ���ᣮ
����Ӧ��Ϻ��ٲ���ƿ�ͷ�Ӧ�����������������Ӧǰ����������Ϊ����������������
��С����С�����������Ľ����飺�����������е���ƿ�ϼ�һװ�и�����ĸ���ܣ��ⶨ��Ӧǰ��װ�õ����������˵��С�����иĽ��������ǣ� ��
�ۻ���ͬѧΪ�˲ⶨ��ͭ��Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼�����
�Լ��㣺
���������ڵ�1����Ʒ��õ������У� �����������ƣ���ȫ��Ӧ�ˣ�
������ͼC�л�����50.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
���Լ����ͭм��Ʒ�е�п��������������д��������̣�����������һλС������

��С������Ƴ�����ͼA�����ⶨ��
������ͭ��Ʒ����ƿ��ϡ�����������
��ͭ��Ʒ������ƿ������ϡ���ᣮ
����Ӧ��Ϻ��ٲ���ƿ�ͷ�Ӧ�����������������Ӧǰ����������Ϊ����������������
��С����С�����������Ľ����飺�����������е���ƿ�ϼ�һװ�и�����ĸ���ܣ��ⶨ��Ӧǰ��װ�õ����������˵��С�����иĽ��������ǣ�
�ۻ���ͬѧΪ�˲ⶨ��ͭ��Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼�����
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 50.0 | 50.0 | 50.0 | 50.0 |
| ȡϡ����������g�� | 40.0 | 80.0 | 120.0 | 160.0 |
| ��������������g�� | 0.4 | 0.8 | 1.0 | 1.0 |
���������ڵ�1����Ʒ��õ������У�
������ͼC�л�����50.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
���Լ����ͭм��Ʒ�е�п��������������д��������̣�����������һλС������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
����������������������ͬʱ���״���������ˮ���������Ӹ���ܵ�ԭ��
��I����һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II���ҳ���㣬���ֵ�����ߵ����ƻ���ͼ�ɣ�
III���Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ�Ȼ��д����ѧ����ʽ������п��������H2SO4��������
��I����һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II���ҳ���㣬���ֵ�����ߵ����ƻ���ͼ�ɣ�
III���Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ�Ȼ��д����ѧ����ʽ������п��������H2SO4��������
����⣺����Ϊ�������ݳ�����ͬʱ��ˮ����Ҳ���������ݳ���������ǰ������������Ҳ����������������ˮ������������
��I��һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II��㣺��û���������ʱ����û������ķų����������ǣ�0��0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣�ÿ����40�����ᣬ����ͻ��ų�0.4�ˣ����Ҫ��ų�1.0�����壬��Ҫ���������������100�ˣ������ߵ��ǣ�100��1.0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣����������������������ı�ֵ��ȣ�б����ͬ��������ͼ����һ��ֱ�ߣ�
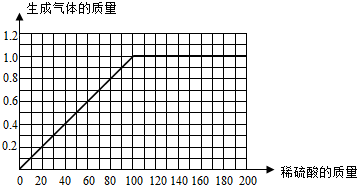
III�Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ���μӷ�Ӧ��п������Ϊx��
��μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 1.0 g
=
x=32.5g
���ͭм��Ʒ�е�п����������Ϊ��
��100%=65.0%
�𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
�ʴ�Ϊ��
�ڷ�Ӧ��������ˮ�������������ݳ���
�ۢ����
��

III���𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
��I��һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II��㣺��û���������ʱ����û������ķų����������ǣ�0��0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣�ÿ����40�����ᣬ����ͻ��ų�0.4�ˣ����Ҫ��ų�1.0�����壬��Ҫ���������������100�ˣ������ߵ��ǣ�100��1.0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣����������������������ı�ֵ��ȣ�б����ͬ��������ͼ����һ��ֱ�ߣ�

III�Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ���μӷ�Ӧ��п������Ϊx��
��μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 1.0 g
| 65 |
| x |
| 2 |
| 1.0g |
x=32.5g
���ͭм��Ʒ�е�п����������Ϊ��
| 32.5g |
| 50g |
�𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
�ʴ�Ϊ��
�ڷ�Ӧ��������ˮ�������������ݳ���
�ۢ����
��

III���𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
�������������йؽ���֪ʶ�Ŀ����⣬��Ŀ���漰��֪ʶ���Լ�Ӧ�ý϶࣬�ܹ��ϺõĶ����֪ʶ����ѵ���Ϳ��飮

��ϰ��ϵ�д�
�����Ŀ
����������������Һ������ӦHCl+NaOH�TNaCl+H2O���˷�Ӧ���ڣ�������
| A�����Ϸ�Ӧ | B���ֽⷴӦ |
| C���û���Ӧ | D�����ֽⷴӦ |
����ʵ������������ȷ���ǣ�������
| A����˿�ڿ�����ȼ�գ��������䣬���ɺ�ɫ���� |
| B������������ȼ�գ���������ɫ���� |
| C����ʢ��Ũ������Լ�ƿ��ƿ����ƿ�ڳ��ְ��� |
| D������������ͨ����ɫʯ����Һ�У���Һ���� |
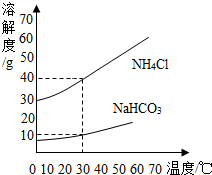 1926�꣬�ҹ�������ѧ�Һ�°�����������һ�ָ�Ч�����Ĵ������������������Ƽ�����ֳƺ����Ƽ�������������������з�Ӧ��
1926�꣬�ҹ�������ѧ�Һ�°�����������һ�ָ�Ч�����Ĵ������������������Ƽ�����ֳƺ����Ƽ�������������������з�Ӧ��
