��Ŀ����
12��ijͬѧ���ⶨij������Ʒ�������Ȼ��ƣ��������������ʣ���̼���Ƶ�������������ѧС���ͬѧ���ʵ�飬��ʵʩʵ�飬��¼�������±���ʾ����Ӧ����ʽΪ��Na2CO3+2HCl�T2NaCl+CO2��+H2O ��| ���ձ������� | ʵ��ǰ��Ʒ���ձ��������� | ����ϡ��������� | ��Ӧ���ձ���ʣ����������� |
| 100.2g | 112.2g | 50g | 157.8g |
��2������Ʒ��̼���Ƶ�����������
���� ��1������̼���������ᷴӦ�����˶�����̼���壬�������ٵ������������ɵĶ�����̼��������
��2�����ݶ�����̼���������̼���Ƶ��������������Ʒ��̼���Ƶ���������
��� �⣺��1���������֪�����ɵĶ�����̼������Ϊ��100.2g--112.2g+50g-157.8g=4.4g
��2������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 44
x 4.4g
$\frac{106}{44}=\frac{x}{4.4g}$ ��ã�x=10.6g
�������Ʒ������=112.2g-100.2g=12g
��Ʒ��̼���Ƶ���������Ϊ��$\frac{10.6g}{12g}��100%$��88.3%��
�ʴ�Ϊ����1��4.4����2������Ʒ��̼���Ƶ�����������88.3%��
���� ����������ͼ���ķ�ʽ�������ͽ����ѧ�����е��й����⣬Ҫ��ѧ���н�ǿ��ʶͼ���������ݷ���������

��ϰ��ϵ�д�
�����Ŀ
13��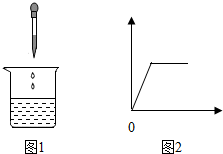 ��ʢ��ij��Һ���ձ�����μ���X��Һ��������ͼ1�������ɳ���������������������꣩�����X��Һ�������������꣩��ϵ������ͼ2���ǣ�������
��ʢ��ij��Һ���ձ�����μ���X��Һ��������ͼ1�������ɳ���������������������꣩�����X��Һ�������������꣩��ϵ������ͼ2���ǣ�������
 ��ʢ��ij��Һ���ձ�����μ���X��Һ��������ͼ1�������ɳ���������������������꣩�����X��Һ�������������꣩��ϵ������ͼ2���ǣ�������
��ʢ��ij��Һ���ձ�����μ���X��Һ��������ͼ1�������ɳ���������������������꣩�����X��Һ�������������꣩��ϵ������ͼ2���ǣ������� | �ձ��е����� | X��Һ | |
| A | ϡ�����ϡ���� | �Ȼ�����Һ |
| B | ϡ���������ͭ��Һ | ����������Һ |
| C | ����ʯ��ˮ | ̼������Һ |
| D | ̼��������Һ | ϡ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
7��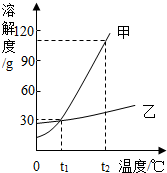 �ס������ֹ������ʵ��ܽ��������ͼ��ʾ������˵��������ǣ�������
�ס������ֹ������ʵ��ܽ��������ͼ��ʾ������˵��������ǣ�������
 �ס������ֹ������ʵ��ܽ��������ͼ��ʾ������˵��������ǣ�������
�ס������ֹ������ʵ��ܽ��������ͼ��ʾ������˵��������ǣ�������| A�� | t1��ʱ���ס��ҵ��ܽ����� | |
| B�� | �ס����У����ܽ�����¶ȵ�Ӱ��ϴ� | |
| C�� | t2��ʱ�����ܽ�ȴ����ҵ��ܽ�ȼ� | |
| D�� | t2��ʱ����100 gˮ�м���90 g�ף��γɱ�����Һ |
1�������йػ�ѧ�������е�Ӧ�ú������ǣ�������
| A�� | �ð״׳��ý��ݳ�ȥ����Ʒˮ���е�ˮ�� | |
| B�� | ��ȼ�շ�������ë��ά�ͺϳ���ά | |
| C�� | �û���̿��������˿ɽ�Ӳˮת������ˮ | |
| D�� | ��ˮ��������Ż� |
 �ƵĻ��������ճ��������й㷺��Ӧ�ã�
�ƵĻ��������ճ��������й㷺��Ӧ�ã�


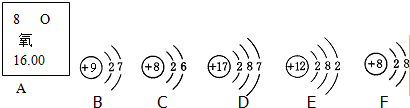
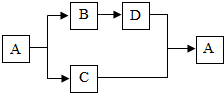 A-D���dz��л�ѧ�г��������ʣ�A��һ��������ˮ�ĸ��Σ���������Ԫ����ɣ�������֮������ͼ��ʾ��ת����ϵ����Ӧ������������Ӧ�P������������ȥ����
A-D���dz��л�ѧ�г��������ʣ�A��һ��������ˮ�ĸ��Σ���������Ԫ����ɣ�������֮������ͼ��ʾ��ת����ϵ����Ӧ������������Ӧ�P������������ȥ����