��Ŀ����
11��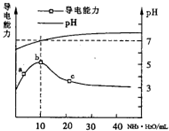 ��������10mL0.1mol/L��HR��Һ����ε���0.1 mol/L��NH3•H2O��Һ��
��������10mL0.1mol/L��HR��Һ����ε���0.1 mol/L��NH3•H2O��Һ��������ҺpH�������Ա仯��ͼ�����з�������ȷ���ǣ�������
| A�� | a��b�㵼��������ǿ˵��HRΪ���� | |
| B�� | b����ҺpH=7˵��NH4Rû��ˮ�� | |
| C�� | c ����Һ����c��NH4+����c��R-����c��OH-����c��H+�� | |
| D�� | b-c�������Һ����c��H+����c��OH-��=KW=1.0��10-14 |
���� A������ͼ���֪��a��b�㵼��������ǿ��˵��HRΪ������ʣ�����Һ�в��ֵ��룬���백ˮ������ǿ����ʣ�����Ũ������
B��b����ҺpH=7˵��笠����Ӻ�R-��ˮ��̶���ȣ�
C��c��ʱ��Һ��pH��7�����Һ��ʾ���ԣ���c��OH-����c��H+������ϵ���غ��֪��c��NH4+����c��R-����
D��b-c�㣬��Һ���¶Ȳ��䣬��ˮ�����ӻ����䣮
��� �⣺A��a��b�㵼��������ǿ��˵����Ӧ����Һ������Ũ������Ҳ֤��HR����Һ�в��ֵ��룬Ϊ���ᣬ��A��ȷ��
B��NH4RΪ���������Σ�NH4R����ҺpH=7��˵��笠����Ӻ�R-��ˮ��̶���ȣ���B����
C������ͼ���֪��c��ʱ��Һ��pH��7�����Һ�ʼ��ԣ���c��OH-����c��H+������ϵ���غ��֪��c��NH4+����c��R-������C��ȷ��
D��������ˮ�����ӻ�Ϊ��KW=c��H+����c��OH-��=1.0��10-14������b-c����Һ�ķ�Ӧ�¶���ͬ����ˮ�����ӻ����䣬��D��ȷ��
��ѡB��
���� ���⿼��������ϵĶ����жϡ�����Ũ�ȶ��ԱȽϡ���Һ�����Լ���Ӱ���֪ʶ����Ŀ�Ѷ��еȣ���ȷ����ϵĶ����жϼ���Һ���������ҺpH�ļ��㷽��Ϊ���ؼ���������ؿ���ѧ���ķ���������������

| A�� | ��Һ�еμ������ữ��BaCl2��Һ���ֱ�ɫ����������Һ�в�һ������SO${\;}_{4}^{2-}$ | |
| B�� | �γ��������������Ҫ��SO2�͵��������CO2���������ЧӦ����Ҫ���� | |
| C�� | ����й©�Ծȷ�������ʪë����պ�д���ˮ��ë����ס�ڱDz�����Ƶ͵ĵط����� | |
| D�� | �����е�N2��ת��ΪNO��NO��ת��ΪNO2������ȣ�������������Ȼ���п�ת��ΪSO2�Ⱥ����� |
| A�� | CH4��C2H4 | B�� | CH4��C3H6 | C�� | C2H4��C3H4 | D�� | C2H2��C3H6 |
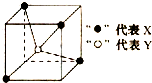
| A�� | YX4 | B�� | XY2 | C�� | YX | D�� | YX2 |
| A�� | N2 | B�� | NO | C�� | NH3 | D�� | NO2? |
| A�� | ��CH2=CH2�����У��������s-sp2�Ҽ���һ���м� | |
| B�� | N��O��F�縺�Դ�С��F��O��N����һ�����ܴ�С��F��O��N | |
| C�� | ����ǿ����H2SO4��H2SO3��H2SeO3���ҽ��������Ⱥ�˳��SiO2��MgSiO3��CaSiO3 | |
| D�� | �ڹ��ۻ������У�һ�����ڼ��Թ��ۼ������ܴ��ڷǼ��Թ��ۼ���һ�����������Ӽ� |