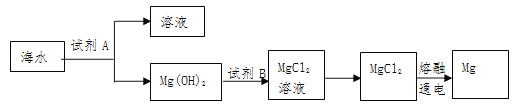
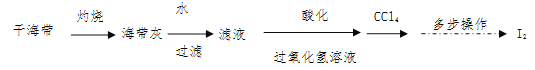��Ŀ����
����Ŀ��һ�ȼ���(CH3C1)��һ����Ҫ�Ļ���ԭ�ϣ�������������ɫ�ж����壬����ˮ���������Ҵ���CCl4�ȡ�
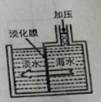
��1������ͬѧ��ʵ��������ͼ��ʾװ��ģ������Ʊ����ռ�һ�ȼ��顣
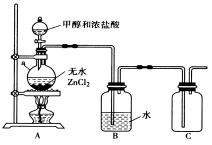
�� ��ˮZnCl2Ϊ������aƿ�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________��
�� װ��B����Ҫ������____________________��
���ռ�����CH3Cl�����������г��ȼ�գ������ù�����V1mL��c1mol��L-1NaOH��Һ������գ��Լ�����ָʾ������c2 mol��L-1�����Һ������Һ���з��ζ�(�����ķ�ӦΪ��
NaOH+HCl= NaCl+H2O ,Na2CO3+2HCl��2NaCl+2CO2��+2H2O)����������V2 mL���ᡣ�����ռ�CH3Cl�����ʵ���Ϊ_____ _ mol��(��֪��2CH3Cl+3O2![]() 2CO2+2H2O+2HCl)
2CO2+2H2O+2HCl)
��2������ͬѧѡ�ü���A��Bװ�ú���ͼ��ʾ������װ�ü���CH3Cl�е���Ԫ�ء�
(��֪��һ±����һ��Ҫ�ڼ��������²���������������Һ��Ӧ)
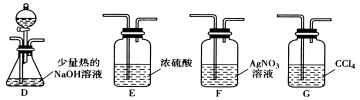
������ͬѧ��ʵ��װ���У��������ӵĺ���˳��ΪA��B��________��__ �� ��
��ͨ��һ��ʱ���CH3Cl���壬��װ��D�з�Һ©���Ļ������۲�ʵ������Һ©����ʢ�ŵ��Լ���_______��_________��
����֤��CH3Cl�к�����Ԫ�ص�ʵ��������_____________________________��
��3���������Ͽ�֪��AgNO3���Ҵ���Һ���Լ���CH3X�е�±��ԭ�ӡ�����������±���

��CH3Xͨ��AgNO3���Ҵ���Һ�У����г��������⣬�����������������д����������Ľṹ��ʽ��______________________________��
����CH3Cl��CH3Br�Ļ������ͨ��AgNO3���Ҵ���Һ�У��ȳ��ֵ���ɫ�����������ݱ������ݽ���ԭ��____________________________________��
���𰸡�
��1����![]()
����ȥHCl���壨1�֣�
����c1V1-c2V2��*10-3��2�֣�
��2����F��D��G ��2�֣�
������ ��������Һ��2�֣�
��F���ް�ɫ�������ɣ�D���а�ɫ�������ɣ�2�֣�
��3����CH3ONO2��1�֣�
��C��Br���ļ���С���������ѣ�Ksp(AgCl)>Kp(AgBr)��AgBr�����׳�����2�֣�
��������
�����������1�����״���Ũ�������Ȼ����������������·���ȡ����Ӧ����һ�ȼ����ˮ����Ӧ�Ļ�ѧ����ʽΪCH3OH+HCl=CH3Cl+H2O���Ȼ�п����ʱ�������ˮ������������п������п����ѧʽΪZn��OH��2��ZnO���ʴ�Ϊ��CH3OH+HCl=CH3Cl+H2O��Zn��OH��2��ZnO��
���Ȼ����ӷ�������Ҫ��ˮ��ȥһ�ȼ����е��Ȼ��⣬����װ��B����Ҫ�����dz�ȥ�Ȼ������壬�ʴ�Ϊ����ȥ�Ȼ������壻���������⣬���Ȼ��ⷴӦ���������Ƶ����ʵ���Ϊc1V1��10-3mol-c2V2��10-3mol������һ�ȼ���ȼ�ղ����Ȼ�������ʵ���Ϊc1V1��10-3mol-c2V2��10-3mol����һ�ȼ�������ʵ���Ϊ��c1V1-c2V2����10-3mol���ʴ�Ϊ����c1V1-c2V2����10-3����2���������⣬����CH3Cl�е���Ԫ�أ����Խ�һ�ȼ���ͨ�뵽�ȵ�����������Һ�У���Ӧһ��ʱ����ټ����������ữ����������Һ�������Ƿ��а�ɫ�������ɿ��ж���Ԫ�صĴ��ڣ�����������ķ�����֪��װ���������ӵĺ���˳��ΪA��B��F��D��G���ʴ�Ϊ��F��D��G������������ķ�����֪����Һ©����ʢ�ŵ��Լ������ᡢ���������ʴ�Ϊ�����ᡢ������������֤��CH3Cl�к�����Ԫ�ص�ʵ��������F���ް�ɫ�������ɣ�D���а�ɫ�������ɣ��ʴ�Ϊ��F���ް�ɫ�������ɣ�D���а�ɫ�������ɣ���3���������⣬CH3Xͨ��AgNO3���Ҵ���Һ�У����������������AgX�����ݳ������ܽ�ƽ���֪���ܶȻ�������ʿ���ת�����ܶȻ�С�ģ�����������Ľṹ��ʽΪ��CH3ONO2���ʴ�Ϊ��CH3ONO2��
�����ݱ������ݿ�֪��C-Br���ļ���С���������ѣ�ͬʱ�廯�����ܶȻ�������С���廯���������γɳ����������ȳ��ֵ���ɫ�廯���������ʴ�Ϊ��C-Br���ļ���С���������ѣ�ͬʱ�廯�����ܶȻ�������С���廯���������γɳ�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���ס�����ͬѧ�Ա����Ѿõ�Na2SO3�Լ���������̽����ȡ����Na2SO3��Ʒ�ڽྻ�ձ��У�������������ˮ����ֽ���ȫ���ܽ⡣
��1����������Һ��PHֵ����PHֵ��7��ԭ���ǣ������ӷ���ʽ��ʾ��
��2��ȡ����������Һ���Թ��У��������ᱵ��Һ���ɰ�ɫ�������ټ������ᣬ��ɫ�������ܽ⣬����Ϊ�ѱ��ʣ�����Ϊ���Ľ��۲���ѧ��������
��3�������Լ���Na2SO3��Na2SO4�е�һ�ֻ�������ɣ�������鷽�������гɷּ��飬���ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�Լ���������ϡ���ᡢϡ���ᡢ�Ȼ�����Һ��Ʒ����Һ�����Ը�������Һ��NaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���á� | |
����2��ȡ��������1������Һ���Թ��У��������������Ը�������Һ��Һ�� | �����Ը��������Һ��ɫ��˵���Լ����� �� ����֮���ޡ� |
����3����ȡ��������1������Һ����һ�Թ��У��ȼ���������ϡ���ᣬ�ٵμ��� ��Һ�� | ����а�ɫ�������ɣ���˵��������Na2SO4���Ѿ����ʣ����û�а�ɫ�������ɣ���˵������û��Na2SO4�� |
��4����ʵ������ȷʵ�ѱ��ʣ���ȷ�ⶨ����Na2SO3�ĺ�����ʵ�����£�
������250ml Լ0��2molL-1 Na2SO3��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�ܽ⣬����Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
�ڵζ���ȷ��ȡ25��00ml Na2SO3��������Һ����ƿ�У���0��05molL-1���Ը������װ��50ml ������ʽ���ʽ���ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ������KMnO4VmL��
�ۼ���Na2SO3����������= ��ֻ�г�����ʽ����Ҫ����������