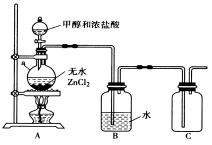
��Ŀ����
����Ŀ���ס�����ͬѧ�Ա����Ѿõ�Na2SO3�Լ���������̽����ȡ����Na2SO3��Ʒ�ڽྻ�ձ��У�������������ˮ����ֽ���ȫ���ܽ⡣
��1����������Һ��PHֵ����PHֵ��7��ԭ���ǣ������ӷ���ʽ��ʾ��
��2��ȡ����������Һ���Թ��У��������ᱵ��Һ���ɰ�ɫ�������ټ������ᣬ��ɫ�������ܽ⣬����Ϊ�ѱ��ʣ�����Ϊ���Ľ��۲���ѧ��������
��3�������Լ���Na2SO3��Na2SO4�е�һ�ֻ�������ɣ�������鷽�������гɷּ��飬���ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�Լ���������ϡ���ᡢϡ���ᡢ�Ȼ�����Һ��Ʒ����Һ�����Ը�������Һ��NaOH��Һ������ʯ��ˮ��pH�ơ��ձ����Թܡ����������ܡ��ι�
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���á� | |
����2��ȡ��������1������Һ���Թ��У��������������Ը�������Һ��Һ�� | �����Ը��������Һ��ɫ��˵���Լ����� �� ����֮���ޡ� |
����3����ȡ��������1������Һ����һ�Թ��У��ȼ���������ϡ���ᣬ�ٵμ��� ��Һ�� | ����а�ɫ�������ɣ���˵��������Na2SO4���Ѿ����ʣ����û�а�ɫ�������ɣ���˵������û��Na2SO4�� |
��4����ʵ������ȷʵ�ѱ��ʣ���ȷ�ⶨ����Na2SO3�ĺ�����ʵ�����£�
������250ml Լ0��2molL-1 Na2SO3��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�ܽ⣬����Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
�ڵζ���ȷ��ȡ25��00ml Na2SO3��������Һ����ƿ�У���0��05molL-1���Ը������װ��50ml ������ʽ���ʽ���ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ������KMnO4VmL��
�ۼ���Na2SO3����������= ��ֻ�г�����ʽ����Ҫ����������
���𰸡���1��SO32-+H2O![]() HSO3-+OH-��2����
HSO3-+OH-��2����
��2����Һ�е�NO3-��H+��һ����ǿ�����ԣ���������ᱵ���������ᱵ��2�֣�
��3����Na2SO3��2�֣����Ȼ�����Һ��2�֣�
��4����250mL����ƿ ��1�֣�����ʽ��1�֣� ��5��0��05��10��126V/2000W��100%��2�֣�
��������
�����������1��Na2SO3 Ϊǿ�������Σ�����ˮSO32��ˮ�⣬��Һ�ʼ��ԣ�pH>7�����ӷ���ʽΪSO32-+H2O![]() HSO3-+OH- ��
HSO3-+OH- ��
��2������Na2SO3 ��Ʒ�Ƿ����, ʵ���Ǽ����Ƿ�����Na2SO4����������Ʒ��Һ���Ƿ����SO42������������������Һ���ټ����ᣬ��Һ�е�NO3����H����һ������ǿ�����������������������������������ȷ����Ʒ�Ƿ��������Na2SO4��
��3�������Լ���Na2SO3��Na2SO4�е�һ�ֻ�������ɣ����гɷּ���ʵ���Ǽ���SO32����SO42���Ƿ���ڣ�SO32������ǿ��ԭ������ʹ���Ը��������Һ��ɫ���������Ը�������Һ��ɫ, ˵����Ʒ����Na2SO3�������Ը�������Һ����ɫ, ˵����û��Na2SO3��SO42�����鷽��Ϊ�ȼ���������ϡ���������μ��Ȼ�����Һ���Ƿ��а�ɫ��������������а�ɫ���������� ��˵����������Na2SO4�����û�а�ɫ������������˵��û��Na2SO4��
��4��������250ml Լ0��2molL-1 Na2SO3��Һ��Ӧѡ��250mL����ƿ����������Ϊ��ȷ��ȡw�������������ձ��У�����������ˮ�ܽ⣬����Һת��250mL����ƿ��ϴ�ӣ����ݣ�ҡ�ȡ������Ը��������Һ����ǿ�����ԣ��ܸ�ʴ��Ӧ����ʽ�ζ���ʢװ����Na2SO3�����Ը��������Һ��Ӧ��Na2SO3������Ϊ�����ƣ�������ر���ԭΪMn2�������ݵ����غ�Ĺ�ϵʽ��5Na2SO3��2KMnO4��250mL��Ʒ��Һ�к��������Ƶ����ʵ���Ϊ10��5��0.05mol/L��10-3VL��2������Ϊ10��5��0.05mol/L��10-3VL��2��126g/mol��Na2SO3����������Ϊ5��0��05��10��126V/2000W��100%��