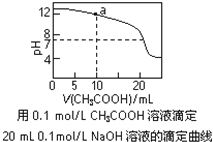
��Ŀ����
����Ŀ��������������ȷ���ǣ� ��
A.0.1molL-1NaOH��Һ��0.2molL-1CH3COOH��Һ�������Ϻ�c(CH3COOH)+c(CH3COO-)=0.1molL-1
B.�����£���CH3COONa��HCl����Һ��ϳ����Ե���Һ�У�c(Na+)>c(Cl-)=c(CH3COOH)
C.pH��ȵĢ�NH4Cl����(NH4)2SO4����NH4HSO4��Һ��c(NH4+)��С˳��Ϊ��=��>��
D.25��ʱ��pH=a��������pH=b��Ba(OH)2��Һ�������Ϻ�ǡ����ȫ��Ӧ����a+b=13
���𰸡�D
��������
A.���������غ������B.���ݵ���غ���з�����C.����Ӱ��ˮ�ĵ������ؽ��з�����D.���ݵ��������кͺ���ҺŨ�ȵļ�����н��
A��![]() ��NaOH��Һ��
��NaOH��Һ��![]() �Ĵ�����Һ�������Ϻ����û����Һ�е�������
�Ĵ�����Һ�������Ϻ����û����Һ�е�������![]() ��
��![]() ��
��![]() ��
��![]() �����������غ��֪��
�����������غ��֪��![]()
![]() ����A��ȷ��
����A��ȷ��
B�����ڻ�Ϻ�����ԣ��ʼ����HCl����ԶС��![]() ��������
��������![]() ��������Һ�е������У�
��������Һ�е������У�![]() ��
��![]() ��NaCl�����ݵ���غ��У�
��NaCl�����ݵ���غ��У� ![]() ��������Һ�����ԣ����У�
��������Һ�����ԣ����У�![]() ���Ӷ���֪��c(Na+)=c(CH3COO-)+c(Cl-)�٣�
���Ӷ���֪��c(Na+)=c(CH3COO-)+c(Cl-)�٣�
��������Һ�����е�![]() ��
��![]() ��
��![]() ��������
��������![]() ��֪��c(Na+)=c(CH3COO-)+c(CH3COOH)�ڣ����
��֪��c(Na+)=c(CH3COO-)+c(CH3COOH)�ڣ����![]() ��֪
��֪![]() ����B��ȷ��
����B��ȷ��
C��![]() ��
��![]() ����ǿ�������Σ���Һ�����Ե�ԭ����NH4+ˮ�⣬����ҺpH��ͬ��˵��������Һ��
����ǿ�������Σ���Һ�����Ե�ԭ����NH4+ˮ�⣬����ҺpH��ͬ��˵��������Һ��![]() Ũ����ȣ�����
Ũ����ȣ�����![]() ����ʱ����
����ʱ����![]() ʹ��Һ�����ԣ�
ʹ��Һ�����ԣ�![]() ��ˮ�ⱻ���ƣ����
��ˮ�ⱻ���ƣ����![]() ��
��![]() ��Ũ��С��
��Ũ��С��![]() ��
��![]() ��С˳��Ϊ
��С˳��Ϊ![]() ����C��ȷ��
����C��ȷ��
D��![]() ��������������Ũ��Ϊ
��������������Ũ��Ϊ![]() ��
��![]() ������������Һ������������Ũ��Ϊ��
������������Һ������������Ũ��Ϊ��![]() ������Һ�������Ϻ�ǡ����ȫ��Ӧ���������Ӻ������������ʵ�����һ����ȣ�����
������Һ�������Ϻ�ǡ����ȫ��Ӧ���������Ӻ������������ʵ�����һ����ȣ�����![]() ����
����![]() ����D����
����D����
�𰸣�D��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���¶�ΪTʱ���������ݻ���Ϊ1L�ĺ����ܱ������н�������Ӧ��2SO2(g)��O2(g)![]() 2SO3(g) ��H��0���ﵽƽ��ʱ������˵������ȷ����
2SO3(g) ��H��0���ﵽƽ��ʱ������˵������ȷ����
���� ��� | �������� | ��ʼ���ʵ��� / mol | ƽ��ʱSO3�����ʵ��� / mol | ||
SO2 | O2 | SO3 | |||
I | ���º��� | 2 | 1 | 0 | 1.8 |
II | ���º�ѹ | 2 | 1 | 0 | a |
III | ���Ⱥ��� | 0 | 0 | 2 | b |
A. ����I��SO2��ת����С������II��SO2��ת����
B. ����II��ƽ�ⳣ����������III�е�ƽ�ⳣ��
C. ƽ��ʱSO3�����ʵ�����a��1.8��b��1.8
D. ����ʼʱ������I�г���0.10 mol SO2(g)��0.20mol O2(g)��2.0 mol SO3(g)�����ʱv����v��