��Ŀ����
11����H��N��O��Na��Cl���ֶ���������Ԫ�أ���1��д�������ӵ�ԭ�ӽṹʾ��ͼ
 ��д��ˮ���ӵĵ���ʽ
��д��ˮ���ӵĵ���ʽ ��
����2��NH4NO3�����ӻ��������ӡ����ۡ�����
��3���������ж����壬д���ñ�������������Һ�������������ӷ���ʽ��Cl2+2OH-=Cl?+ClO-+H2O��
��4������Ԫ��������ѧ��ѧ����Ԫ�أ��������ʽ����Ȼ�����Һ�У��÷�Ӧ�����ӷ���ʽΪFe+2Fe3+=3Fe2+��
��5�������ݣ�4���еķ�Ӧ�����һ��ԭ��أ�Ҫ����ʵ��װ��ͼ��ע���������Һ���ơ������������������ϣ�����������ƶ�����д���缫��Ӧʽ��
������Ӧʽ��2Fe3++2e-=2Fe2+��
������Ӧʽ��Fe-2e-=Fe2+��
���� ��1��������ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ8��ˮΪ���ۻ����
��2��NH4NO3Ϊ���ӻ����
��3���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��4�������Ȼ�����Ӧ�����Ȼ�������
��5���γ�ԭ��ط�Ӧʱ����Ϊ������������Ϊ̼�����������ҺΪ�Ȼ����������������������������ӱ���ԭ��
��� �⣺��1��������ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ8�����ӽṹʾ��ͼΪ ��H2O�ǹ��ۻ��������ԭ�Ӻ���ԭ���γɹ��ۼ�������ʽΪ
��H2O�ǹ��ۻ��������ԭ�Ӻ���ԭ���γɹ��ۼ�������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��2��NH4NO3Ϊ��Σ��������ӻ�����ʴ�Ϊ�����ӣ�
��3���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽ��Cl2+2OH-=Cl?+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-=Cl?+ClO-+H2O��
��4�������Ȼ�����Ӧ�����Ȼ���������Ӧ�����ӷ���ʽΪFe+2Fe3+=3Fe2+���ʴ�Ϊ��Fe+2Fe3+=3Fe2+��
��5��ʯī--�����Ȼ����������Һ���γ�ԭ��أ�ʯīΪ������Fe3+�������ϵõ��ӱ���ԭ���缫��ӦʽΪ��2Fe3++2e-=2Fe2+�������������缫��ӦʽΪFe-2e-=Fe2+�������ɸ���������������������ԭ���װ��ͼΪ��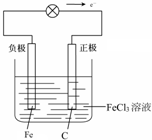 ��
��
�ʴ�Ϊ��2Fe3++2e-=2Fe2+��Fe-2e-=Fe2+�� ��
��
���� �����ۺϿ���Ԫ�ػ������ԭ��ء����û�ѧ���Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ�ע��ԭ���װ��ͼ�Ļ滭�Լ�ԭ��صĹ���ԭ��������������ʵ����ʣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | n=m+1 | B�� | n=m+10 | C�� | n=m+11 | D�� | n=m+25 |
| A�� | ʹ pH ��ֽ�ʺ�ɫ����Һ��Fe2+��NH4+��Cl-��NO3- | |
| B�� | pH=13����Һ��S2-��SO32-��SO42-��Na+ | |
| C�� | ʹ��ɫʯ����ֽ����ɫ����Һ��K+��HCO3-��Br-��Ba2+ | |
| D�� | ��������ΪK2SO4����Һ��AlO2-��Na+��Al3+��Ba2+ |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | �� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� |
��2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4����Ԫ�ط��ţ���
��3��Ԫ�آڵ���̬�⻯��������ڷǵ���ʣ������ʻ�ǵ���ʣ���Ԫ�آ�����γɻ�����ĵ���ʽ��
 ��
����4��Ԫ�آݵ����������������������Һ��Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=H2O+2AlO2-��
��5��Ԫ�آܵ�ij��������������������������������Ļ�ѧ�����������Ӽ������ۼ���д�����������̼��Ӧ�Ļ�ѧ����ʽ2Na2O2+2CO2=2Na2CO3+O2��
| A�� | 1 mol Al3+���еĺ��������Ϊ3��6.02��1023 | |
| B�� | ��58.5 g NaCl����1.00 Lˮ�У�����NaCl��Һ��Ũ��Ϊ1.00 mol•L-1 | |
| C�� | 2 mol SO2������������O2��һ�������·�Ӧ��ת�Ƶĵ�����Ϊ4��6.02��1023 | |
| D�� | �����£�100mL pH=1��������Һ�к��е�H+������Ϊ0.01��6.02��1023 |
| A�� | AgCl�ڱ���NaCl��Һ�е�KSP���ڴ�ˮ�е�KSPС | |
| B�� | KSP��AgCl����KSP��Ag2CrO4������˵��Ag2CrO4��AgCl������ˮ | |
| C�� | ��0.00lmol•L-1AgNO3��Һ����0.001 mol•L-1KC1��0.00lmol•L-1K2CrO4�����Һ�У��Ȳ���Ag2CrO4���� | |
| D�� | ͬAgCl������Һ�еμ�Ũ��ˮ������ܽ⣬����Ϊ�������������Ӷ�ʹAgCl���ܽ�ƽ�������ƶ� |
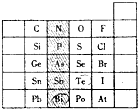 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�