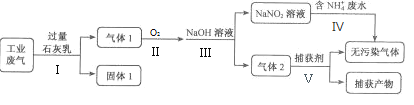
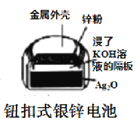
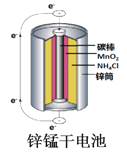
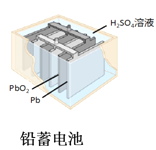
��Ŀ����
����Ŀ��[��ѧ��ѡ��3�����ʽṹ������]
��1��Fe3+�ĵ����Ų�ʽΪ___________________����֪��Fe3���Ļ�ѧ���ʱ�Fe2���ȶ������ԭ�ӽṹ�ĽǶȽ��н���____________________��
��2��Fe����CO�γ������Fe(CO)5��1 mol Fe(CO)5�к���________ mol�Ҽ���
��3����CO��Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ________ ��_______(����һ�֣��ѧʽ)��
��4��ij��������Cu�������ʾ���ϼ�Ϊ+1������γ�ͼ����ʾ�����ӡ��������к��л�ѧ����������____________________��������ţ�
A�����Լ� B�����Ӽ� C���Ǽ��Լ� D����λ��
��5�����Ȼ�ͭ��Һ��ͨ�������Ķ����������ɰ�ɫ����M�� M�ľ����ṹ��ͼ����ʾ��д���÷�Ӧ�����ӷ���ʽ��____________________��
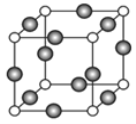
��6����֪��������Ԫ����ɵĻ�����AΪ�������뵼�塣��֪������A�ľ����ṹ����ʯ���ƣ��侧���ṹ��ͼ����ʾ����д��������A�Ļ�ѧʽ___________���軯����A�ľ����߳�Ϊ![]() pm����ÿ�������þ�����������Ԫ�ص�����Ϊ______________g��NA��ʾ�����ӵ�������ֵ����
pm����ÿ�������þ�����������Ԫ�ص�����Ϊ______________g��NA��ʾ�����ӵ�������ֵ����

���𰸡� 1s22s22p63s23p63d5��[Ar]3d5 Fe3����3d��������5�����ӣ�Ϊ�����״̬ 10 N2 CN�� A C D 2Cu2+ + 2Cl- + SO2 + 2H2O �� 2CuCl�� + SO4 2-+ 4H+ GaAs 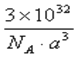
��������
��1�����ݵ����Ų�ʽ��дҪ���ع�����������ԭ���������2������Fe(CO)5�Ľṹ�������ĺ�������������3��ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ��������������4�����ݻ�ѧ��������𣻣�5������M�ľ����ṹ��ͼ���Ʋ�M�Ļ�ѧʽ�����ݷ�Ӧ���������д�����ӷ���ʽ����6�����ݾ����ṹ�Ʋ�A�Ļ�ѧʽ�������м��㡣
��1��Fe����Ԫ�����ڱ�����26��Ԫ�أ�����Fe3+�ĵ����Ų�ʽΪ1s22s22p63s23p63d5��[Ar]3d5��Fe2+�ĵ����Ų�ʽ�ǣ�1s22s22p63s23p63d6�����ݺ��ع���3d5�ǰ����״̬�������ϵͣ��Ƚ��ȶ�����3d6���ǰ����״̬�������ϸߣ�������ʧȥһ�����ӱ�ɰ�������ȶ�״̬��������ȷ����1s22s22p63s23p63d5��[Ar]3d5��Fe3����3d��������5�����ӣ�Ϊ�����״̬��
��2��Fe��COͨ����λ���γ�����������5��������C��Oԭ��֮���γ�������������������1�������������Թ���5������������1 mol Fe(CO)5�к���10 mol��������ȷ����10��
��3�����ݵȵ�����Ķ����֪��CO��2��ԭ�ӣ�14��������ɵ���������ȵ�������N2���Ӻ�CN-���ӣ���ȷ����N2��CN����
��4���������ͼ��֪��Nԭ����Cuԭ��֮���γ���λ����Nԭ����Cԭ��֮�䡢Nԭ����Hԭ��֮�䡢Cԭ����Hԭ��֮���γɼ��Լ���Cԭ����Cԭ��֮���γɷǼ��Լ������Լ��д��ڵĹ��ۼ������м��Լ����Ǽ��Լ�����λ��������ѡ��A��C��D����ȷ�𰸣�A��C��D��
��5����ͼ�ҿ��Կ������ó��������Ӧ��������Ԫ����ɣ��þ��������������Լ���Ϊ��С����4����������8��1/8+6��1/2=4������������Ԫ����������Ϊ1��1���ó������Ȼ�ͭ��Һ��ͨ�������Ķ�������Ӧ���ɣ�Cu2+��ǿ�������ԣ�SO2���л�ԭ�ԣ�������Ӧ����Cu+��SO42-���ɣ��dz���ֻ����Cu+��Cl-��϶��ɵġ����Ը���������ԭԭ����д����Ϊ2Cu2+��2Cl����SO2��2H2O��2CuCl��+SO42��+4H+��
��6���ɾ����ṹ��֪��С����4����������8��1/8+6��1/2=4���û����ﻯѧʽΪGaAs�������������V=��apm��3=a3��10-10cm3��ÿ�������к�4��Asԭ�ӣ�����ÿ�������þ�����������Ԫ�ص�����Ϊ��4��75g��mol-1/NAV=3��1032/a3NA����ȷ�𰸣�GaAs��3��1032/a3NA��

 ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д�����Ŀ��ij̽��С�������������ʯ��Ӧ������������С�ķ������о�Ӱ�췴Ӧ���ʵ����ء�����HClŨ��Ϊ1.00mol/L��2.50mol/L������ʯ��ϸ�����ʹֿ������ֹ��ʵ���¶�Ϊ25�桢40�棬ÿ��ʵ�����������Ϊ25.00mL������ʯ����Ϊ10.00g��
��1��д�����������ʯ��Ӧ�Ļ�ѧ����ʽ________
��2�����������ʵ����Ʊ�������ʵ���п�ȱ������������
ʵ�� ��� | �¶� ���棩 | ����ʯ ��� | HClŨ�ȣ�mol/L�� | ʵ��Ŀ�� |
�� | 25 | �ֿ��� | 2.50 | (I)ʵ��ٺ͢�̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죻 (II)ʵ��ٺ͢�̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죻 (III)ʵ��ٺ͢�̽��______�Է�Ӧ���ʵ�Ӱ�� |
�� | __ | �ֿ��� | 2.50 | |
�� | 25 | �ֿ��� | ___ | |
�� | 25 | ϸ���� | 2.50 |
��3��ʵ�����CO2������ʱ��仯�Ĺ�ϵ����ͼ������ʵ�����70s-90s��Χ����HCl��ʾ��ƽ����Ӧ���� ______��������Һ����仯��

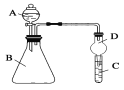
����Ŀ�������ģ�ij��ȤС���������ͼ��ʾװ��(���ּг�װ������ȥ)����ʵ��̽����
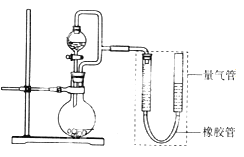
��ʵ��һ��̽��Ӱ�컯ѧ��Ӧ���ʵ����ء�
Բ����ƿ��װпƬ(����ʵ��������пƬ��С��������ͬ)����ѹ��Һ©����װϡ���ᣬ������20.0mL����Ϊ��ʱ�յ㣬���Ϊt1��t2��
��� | V(H2SO4)/mL | c(H2SO4)/mol��L-1 | t/s | ||||
I | 40 | 1 | t1 | ||||
II | 40 | 3 | t2 | ||||
��� | V(H2SO4)/mL | c(H2SO4)/mol��L��1 | t/s | ||||
I | 40 | 1 | t1 | ||||
II | 40 | 3 | t2 | ||||
����װ�������Եķ�����_______________________________________________��
�Ƚ�ʵ��I�͢���Եó��Ľ�����____________________________________________��
��ʵ�����̽�����ĵ绯ѧ��ʴ��
��Բ����ƿ��װ���ۺ�̼�ۻ�����ѹ��Һ©����װϡ���ᣬ��������ϡ������������г��ֵ������ǣ����Һ��_________�Ҳ�Һ��_________(ѡ������������½���)��
��Բ����ƿ��װ�����ͬ�������۵�������̼�ۣ������Լ��Ͳ�����ͬ���������Ҳ�Һ��仯��_______��ѡ��족������������ͬ����˵��ԭ��ط�Ӧ��һ�㻯ѧ��Ӧ_______��
��Բ����ƿ��װ�����ͬ�������ۺ�̼�ۻ�����ѹ��Һ©����װʳ��ˮ����������ʳ��ˮ����Ԥ���������г��ֵ������ǣ�___________________________________�������ĵ缫��Ӧ��___________________________��