题目内容
实验室配制500mL 0.1mol/L的NaOH溶液,有如下操作步骤:
①计算所需NaOH固体的质量并用托盘天平称取;
②将称量好的NaOH固体放入烧杯中,加入适量的蒸馏水溶解,然后转移至容量瓶中;
③用少量蒸馏水洗涤烧杯和玻璃棒2~3次,每次洗涤的液体都小心转入容量瓶中并轻轻摇匀;
④继续向容量瓶中加蒸馏水至液面距刻度线1~2cm处,改用胶头滴管小心滴加蒸馏水至溶液凹液面与刻度线相切;
⑤塞紧容量瓶的塞子,充分摇匀。
回答下列问题:
(1)容量瓶在使用前,必须
(2)实验中用托盘天平实际称取NaOH固体的质量是
(3)上述实验操作②中,缺少的步骤是
(4)在实验中,未进行操作③,所配溶液的浓度会 (填“偏高”、“偏低”或“无影响”,下同);定容时俯视液面,所配溶液的浓度会 。称量前容量瓶有少量水,所配溶液的浓度会 。
(1)检验是否漏水 (2)2.0g (3)冷却 (4)偏低 , 偏高 , 无影响
解析试题分析:(1)容量瓶是准确配制一定体积、一定浓度的溶液的仪器。由于配制的溶液由均一性、稳定性。在配制的最后一步要摇匀。所以在使用前,必须检查容量瓶是否漏水。(2)n(NaOH)="C·V=" 0.1mol/L×0.5L="0.05mol.m(NaOH)=" 0.05mol×40g/mol=2.0g.托盘天平的准确度为0.1g,所以实验中用托盘天平实际称取NaOH固体的质量是2.0g. (3) NaOH固体溶解在水中放出热量,而容量瓶配制溶液时要求的温度是室温20度,所以上述实验操作②中,缺少的步骤是冷却至室温。(4)在实验中,溶解溶质使用的烧杯的内壁及玻璃棒上都沾有溶质,若未进行操作③,就会使一部分溶质没有完全转移到容量瓶中,所以所配溶液的浓度会偏低。定容时如果俯视液面,则加入的溶液的体积就会偏小,则所配溶液的浓度会偏高。如果称量前容量瓶有少量水,但由于未改变溶质的物质的量的多少,也未影响溶液的体积的大小,所以对所配溶液的浓度不会产生任何影响。
考点:考查容量瓶的使用、物质的量浓度的溶液的配制的步骤及误差分析的知识。

下列关于实验的说法正确的是
| A.用玻璃棒蘸取NaOH溶液,滴在pH试纸上,马上和比色卡对照,确定NaOH的pH |
| B.滴定用的滴定管、锥形瓶和配制一定物质的量浓度溶液用的容量瓶,使用前均要润洗 |
| C.均不能采取将溶液直接蒸干的方法制得AlCl3、Al2(SO4)3、FeCl3、Fe2(SO4)3 |
| D.因为Ksp(CaCO3)<Ksp(CaSO4),所以可用Na2CO3和HCl除去水垢中的CaSO4 |
下列有关化学实验的说法中正确的是
| A.烧杯、坩埚、试管、锥形瓶都可以用酒精灯直接加热 |
| B.银镜反应、乙醛与新制Cu(OH)2反应、实验室制取乙烯都必须用水浴加热 |
| C.制取氨气、氢气时都可以用向下排空气法收集 |
| D.石油的分馏、实验室制取乙炔和制取蒸馏水都要用到冷凝装置 |
高锰酸钾是一种重要的化学试剂,其溶液不很稳定,在酸性条件下会分解生成二氧化锰和氧气,在中性或弱碱性溶液中分解速度很慢,见光分解速度加快。
(1)酸性条件下高锰酸钾溶液分解的离子方程式 。
(2)请配平高锰酸钾溶液与草酸钠Na2C2O4溶液在酸性条件下反应的离子方程式:
______MnO4— +______C2O42—+______H+=______Mn2++______CO2↑+____________
(3)某学习小组为了探究高锰酸钾溶液和草酸钠溶液的反应过程,将高锰酸钾溶液逐滴地滴入一定体积的酸性草酸钠溶液中(温度相同,并不断振荡时),记录的现象如下表:
滴入高锰酸钾溶液的次序(每滴溶液的体积相同) | 高锰酸钾溶液紫色褪去的时间 |
| 先滴入第1滴 | 1min |
| 褪色后再滴入第2滴 | 15s |
| 褪色后再滴入第3滴 | 3s |
| 褪色后再滴入第4滴 | 1s |
请分析高锰酸钾溶液褪色时间变化的原因 。
(4)该学习小组在获取了上述经验和结论以后,用稳定的物质草酸钠Na2C2O4(相对分子质量134.0)来标定高锰酸钾溶液的浓度。他们准确称取1.340g纯净的草酸钠配成250mL溶液,每次准确量取25.00mL溶液酸化后用KMnO4溶液滴定。
①高锰酸钾溶液应装在 (填下图中的仪器编号)。
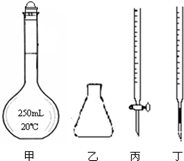
②为了防止高锰酸钾在酸性条件下分解而造成误差,滴定时应注意的是 。
③若在实验过程中存在下列操作,其中会使所测KMnO4浓度偏低的是 。
A.未润洗盛放KMnO4的滴定管
B.滴定前尖嘴部分无气泡,滴定终点时出现气泡
C.定容时,俯视刻度线
D.锥形瓶用水洗之后未用待测液润洗
④当溶液呈微红色且半分钟内不褪色,消耗KMnO4溶液20.00mL(多次测定的平均值),则KMnO4溶液的浓度为 。


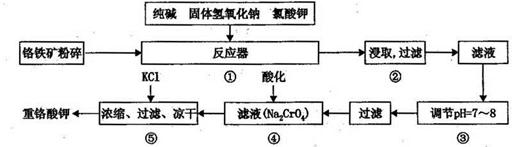
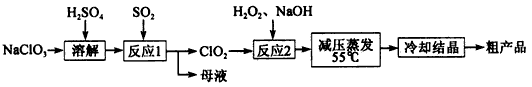
 12Na2CrO4+3Fe2O3+7KCl+12H2O
12Na2CrO4+3Fe2O3+7KCl+12H2O