��Ŀ����
CuSO4��Һ��K2C2O4��Һ��Ӧ���õ�һ����ɫ�ᾧˮ���ᄃ�塣ͨ������ʵ��ȷ���þ������ɣ�
�ٳ�ȡ0.1680g���壬���������H2SO4��Һ��ʹ��Ʒ�ܽ���������ˮ�����Ƚ��У���0.02000mol��L-1KMnO4��Һ�ζ����յ㣨��Һ��Ϊdz�Ϻ�ɫ��������20.00mL��
�ڽ��Ž���Һ��ּ��ȣ�ʹdz�Ϻ�ɫ��Ϊ��ɫ����ʱMnO��4ת��ΪMn2+���ͷų�O2��
����ȴ�����2g KI���壨������������Na2CO3����Һ��Ϊ��ɫ�����ɳ�����
����0.05000mol��L-1Na2S2O3��Һ�ζ������յ��ָʾ�����ζ����յ㣬����10.00mL��
��֪��2MnO��4+5H2C2O4+6H+==2Mn2++10CO2��+8H2O
2Cu2++4I��=2CuI��+I2
2Na2S2O3+I2=2NaI+Na2S4O6
��1��������з�����Ӧ�����ӷ���ʽΪ ��
��2��������м����ָʾ��Ϊ ��
��3��ͨ������д����ɫ����Ļ�ѧʽ��д��������̣���
��1��4MnO4- +12H+=4Mn2++5O2��+6H2O
��2��������Һ
��3��n(C2O42-)=0.02000��20.00��10-3��5/2=1.00��10-3mol
n(Cu2+)=0.05000��10.00��10-3=5.00��10-4mol
���ݵ���غ��֪�����廹����������K+��
n(K+)=2��1.00��10-3-2��5.00��10-4=1.00��10-3mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��xH2O
m(H2O)=0.1680-5.00��10-4��318=0.009g
n(H2O)=0.009/18=5.00��10-4mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��H2O
�����������������ⶨ������ɵ��ۺϼ����⣬(2)�еⵥ�ʲ�������ɵķ�Ӧ�������õ�����Ϊ��Ӧ�Ƿ���ȫ�����ݣ���3�����ݵڢٲ���Ӧ���Լ����C2O42-������n(C2O42-)=0.02000��20.00��10-3��5/2=1.00��10-3mol
�ڢ��dz�ȥ��Һ�ж���ĸ��������Һ���ڢۢܿ��Լ����ͭ���ӵ���
n(Cu2+)=0.05000��10.00��10-3=5.00��10-4mol
���ݵ���غ��֪�����廹����������K+��
n(K+)=2��1.00��10-3-2��5.00��10-4=1.00��10-3mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��xH2O
Ȼ���ٸ���������������ᾧˮ������m(H2O)=0.1680-5.00��10-4��318=0.009g
n(H2O)=0.009/18=5.00��10-4mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��H2O
���㣺�����Թ�ҵ�ⶨ������ɶ���Ƶļ����⣬�漰���ӷ���ʽ��д���ζ�ָʾ��ѡ�����غ������й����⡣

����˵������ȷ����
| A��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ��Ѹ����ʪĨ���˸� |
| B���õ�����ƽ������ѧҩƷʱ�������ȳ�С�ձ����������ٳ��������Լ����������������֮�ΪҩƷ�������� |
| C���Ʊ�Ħ���εĹ����У�ϴ����������茶���ʱ��Ӧ�������ƾ�ϴȥ������渽�ŵ�ˮ�� |
| D���������Ȼ��ܵ��Ҵ���Һ�У���μ���ˮ����Һǡ�óʷۺ�ɫ�����ȸ���Һ��������γ�����ɫ������ɫ����ɫ�ı仯 |
ij��ѧС���ͬѧ��ʵ����ѧϰ����ʵ�����ϰ�����ͼ��ʾ������
(1)ָ����ʦҪ��ͬѧ��д�����������ƣ�ijͬѧ��д�Ĵ����±��������ҳ����еĴ����������������д���±���(����ȷ����˿ղ���Ҫ��д)��
| ������� | a | b | c | d | e |
| ���� | �Թ� | ����ƿ | ����ƿ | ��ʽ�ζ��� | ��ͨ©�� |
| ���������� | | | | | |
(2)��ͬѧ����d����ʵ�飬����˵��������(�Ѿ�ϴ�Ӹɾ�)ʹ��ʱ�ĵ�һ�������� ��
(3)����e����;����ͬѧ˵����ɷ�����װ�á��㻹��˵������������;��
�� ��
�� ��
(4)��ͬѧ������ͼ��ʾװ���ô���ʯ��ϡ���ᷴӦ��ȡCO2����ʦָ��������Ҫ̫���ϡ���ᣬ������˷ѣ���ͬѧѡ���������һ������������װ���У������������⡣����Ѹ���������ͼ�к��ʵ�λ�á�

��ҵ����﮻�ʯ(Li2O��Al2O3��4SiO2��������Ca��MgԪ��)Ϊԭ������̼��ﮡ��䲿�ֹ����������£�
��֪����Li2O��Al2O3��4SiO2 H2SO4(Ũ)
H2SO4(Ũ) Li2SO4
Li2SO4 Al2O3��4SiO2��H2O��
Al2O3��4SiO2��H2O��
��ijЩ���ʵ��ܽ��(S)���±���ʾ����
| T/�� | 20 | 40 | 60 | 80 |
 (Li2CO3)/g (Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
 (Li2SO4)/g (Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |
�۴�����1����ȡ��Si�IJ�����������ͼ��ʾ��

����������Ϣ����ش��������⣺
��1��������Ӧ�ṩ����Ӧ�������������� �������� �� ��������
��2���������������ʵĻ�ѧʽ���� ���� ����
��3��ʹ��Ũ���Ტ���ȵ�250�桫300���Ŀ�������������������� ������
��4���ڴ���Na2CO3��Һ��������Ӧ�����ӷ���ʽ������ �� ���� ���� ��
��5������2����Ҫ�ɷ������������� ��������
��6����μ���Li2CO3�Ƿ�ϴ�Ӹɾ����������� ��������������
ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��
�Ʊ������л���CH3COOH��AlCl3�D��CH3COOAlCl2��HCl���ȸ���Ӧ��
��Ҫʵ��װ�úͲ������£�
(��)�ϳɣ�������ƿ�м���20g��ˮ���Ȼ�����30mL
��ˮ����Ϊ���ⷴӦҺ���¹��죬�߽���������μ�6mL
��������10mL��ˮ���Ļ��Һ�����Ƶμ����ʣ�ʹ��ӦҺ
�����������μ���Ϻ���Ȼ���1Сʱ��
(��)�������ᴿ���ٱ߽���������μ�һ����Ũ�������ˮ���Һ������õ��л����ˮ���ñ���ȡ����Һ�۽��٢������л���ϲ���ϴ�ӡ������ȥ�����õ�����ͪ�ֲ�Ʒ������ֲ�Ʒ�õ�����ͪ
�ش��������⣺(1)����a�����ƣ�________��װ��b�����ã�________��
(2)�ϳɹ�����Ҫ����ˮ������������_______________________��
(3)�����������ͱ��Ļ��Һһ���Ե�������ƿ�����ܵ���________��
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |
(5)��Һ©��ʹ��ǰ��________��ϴ�����á���ȡʱ���Ⱥ�������ȡҺ����ȡ��������ҡ��________����Һ©����������̨����Ȧ�Ͼ���Ƭ�̣��ֲ㡣�������²�Һ��ʱ��Ӧ��________��Ȼ������ų��²�Һ�壬�ϲ�Һ����Ͽڵ�����
(6)�ֲ�Ʒ�����ᴿʱ������װ�����¶ȼ�λ����ȷ����________�����ܻᵼ���ռ����IJ�Ʒ�л��еͷе����ʵ�װ����________��

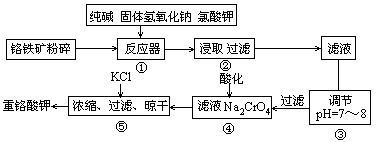
 12Na2CrO4+3Fe2O3+7KCl+12H2O��
12Na2CrO4+3Fe2O3+7KCl+12H2O��