��Ŀ����
һ���¶��£���1.0L�ܱ������м���0.60molX(g)��������ӦX(g)  Y(s)+2Z(g)��H��0��÷�Ӧ��XŨ���뷴Ӧʱ����������±�
Y(s)+2Z(g)��H��0��÷�Ӧ��XŨ���뷴Ӧʱ����������±�
| ��Ӧʱ��t/min | 0 | 1 | 2 | 3 | 4 | 6 | 8 |
| c(X)/(mol��L-1) | 0.60 | 0.42 | 0.30 | 0.21 | 0.15 | a | 0.0375 |
��1��3minʱ��Z��ʾ��ƽ����Ӧ����v(Z)�� ��
��2�������÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ��ɣ��ó��Ľ����� ���ɴ˹����Ƴ���Ӧ��6minʱ��Ӧ���Ũ��aΪ mol��L-1��
��3����Ӧ���淴Ӧ������ʱ��仯�Ĺ�ϵ��ͼ��t2ʱ�ı���ijһ���������ı������������ �� ����д������
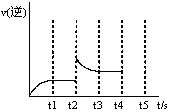
��4��������Щ��������������Ѵ�ƽ��״̬ ������ĸ��ţ�
A���������һ��ʱ�����ܶȲ��ٱ仯
B����Ӧ��ƽ�ⳣ�����ٱ仯
C�������������ƽ����Է�����������ʱ����仯
D��Y�����ʵ������ٷ����仯
E��Z���������ʵ���X���������ʵ�2��
(10��)
��1��0.26mol��L-1��min-1��2�֣�
��2��ÿ���2min��X��Ũ��Ϊԭ����һ�루2�֣���0.075��2�֣�
��3������Z ������ϵ��ѹǿ��2�֣�
��4��ACD��2�֣�
�������������
3min�ǡ�c(X)=0.39mol��L-1��v(X)=0.39mol��L-1/3min=0.13mol��L-1��min-1����2v(X)="v(Z)=" 0.26mol��L-1��min-1��
�����������ݣ��ɼ�ÿ���2min��X��Ũ��Ϊԭ����һ�룻�ɴ˹����Ƴ���Ӧ��6minʱ��Ӧ���Ũ��aΪ0.075 mol��L-1
t2ʱ�̣�v�������ܵ�����Ϊ����������Z��������ϵ��ѹǿ��
A��������Y�ǹ��壬δ����ƽ��ʱ�����������ڱ仯���ܶ�Ҳ�ڱ仯����ƽ���Dz��ٱ仯���ʿ���˵������ȷ��B��ƽ�ⳣ��ֻ���¶��йأ�����C�����������������������ͬ��ֻ��ƽ��ʱ�����ƽ����Է�����������ʱ����仯����ȷ��D��Y�������ڱ仯���ﵽƽ�⣬��ȷ��E��2����Z���������ʵ���X���������ʲ���ƽ��̬������
���㣺���⿼�黯ѧ��Ӧ���ʡ�Ӱ�����ʵ����ء�ƽ������������֪ʶ��

��һ��������ܱ������У��������·�Ӧ��A��g�� B��g��+C��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ��
| toC | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ��ѧƽ�ⳣ���ı���ʽ��K= ��
��2������ͼ����ʵ�����÷�Ӧ�������仯���ߣ�ͬʱ�ڴ˻����������������������������仯���ߡ�

��3��һ���¶Ⱥ�����£�����˵������˵���÷�Ӧ��ƽ��״̬���� ��
��������ѹǿ����
�ڻ��������c��C������
�ۻ��������ܶȲ���
��v��A��=v��B��
�ݻ�ѧƽ�ⳣ��K����
�������ƽ��ʽ������
��4����Ӧʱ�䣨t��������������A��Ũ�����ݼ��±�
| ʱ��t/min | 0 | 1 | 2 | 4 | 8 | 16 | 20 |
| C��A��/��mol��L��1�� | 10.4 | 8.6 | 7.5 | 6.6 | 5.9 | 5.5 | 5.5 |
�ش��������⣺
��2��4min�ڣ�B��ƽ������Ϊ ��
�ڷ�Ӧ��ƽ��ʱ��A��ת����Ϊ ��
�������A��ƽ��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
��ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
��1����֪�ò�ҵ����ij��Ӧ��ƽ�ⳣ������ʽΪ��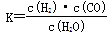 ��������Ӧ��Ӧ�Ļ�ѧ����ʽ��
��������Ӧ��Ӧ�Ļ�ѧ����ʽ��
_________________________________��
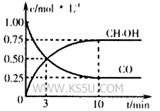
��2���ϳɼ״�����Ҫ��Ӧ�ǣ�2H2��g��+CO��g�� CH3OH��g��+90.8kJ��t���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������п�ʼֻ����CO��H2����Ӧl0min���ø���ֵ�Ũ�����£�
CH3OH��g��+90.8kJ��t���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������п�ʼֻ����CO��H2����Ӧl0min���ø���ֵ�Ũ�����£�
| ���� | H2 | CO | CH3OH |
| Ũ�ȣ�mol/L�� | 0.2 | 0.1 | 0.4 |
�ٸ�ʱ����ڷ�Ӧ����v(H2)=
�ڱȽϴ�ʱ�����淴Ӧ���ʵĴ�С��v�� v�����>������<����=����
�۷�Ӧ�ﵽƽ����������������䣬��ֻ�������������Сһ�룬ƽ���� (�������������)�ƶ���ƽ�ⳣ��K (���������С�� ���䡱)��
��3���̵��ǿ�ѧ�������о�����Ҫ���⡣��Ȼ���д�����Ȼ�Ĵ����̵����̣�N2 (g) + O2 (g) ��2NO (g) ��180.8 kJ����ҵ�ϳɰ������˹��̵����������̵ֹ���Ӧ��ƽ�ⳣ�������н�����ȷ���� ��
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
A�������£������̵����ѽ��У�����ҵ�̵�ȴ�ܷdz�������
B��ģ������̵�Ӧ���ڹ�ҵ�ϵ����岻��
C����ҵ�̵�ʱ�¶�Խ�ͣ�������������ӦԽ��ȫ
D��KԽ��˵���ϳɰ���Ӧ������Խ��
��֪A(g)+B(g)  C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
| �¶�/�� | 700 | 900 | 830 | 1000 | 1200 |
| ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
��1�� 830��ʱ����һ��5 L���ܱ������г���0.20mol��A��0.80mol��B���練Ӧ��ʼ6s��A��ƽ����Ӧ����v(A)="0.003" mol��L-1��s-1����6sʱc(A)��________mol��L-1�� C�����ʵ���Ϊ______ mol����ʱ������Ӧ����_____________������ڡ�����С�ڡ����ڡ����淴Ӧ���ʡ�
��2���ں����ܱ��������жϸ÷�Ӧ�Ƿ�ﵽƽ�������Ϊ________(����ȷѡ��ǰ����ĸ)��
a��ѹǿ����ʱ��ı� b.������ܶȲ���ʱ��ı�
c. c(A)����ʱ��ı� d.��λʱ��������c��D�����ʵ������
��3��1200��ʱ��ӦC(g)+D(g)
 A(g)+B(g)��ƽ�ⳣ����ֵΪ___________________��
A(g)+B(g)��ƽ�ⳣ����ֵΪ___________________����4����������������罻���������ں��ݾ��������£�����2M(g)+N��g��
 2P��g��+Q��s����Ӧ�����±�����Ͷ�ϣ���Ӧ�ﵽƽ��״̬�������ϵѹǿ���ߣ������÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��ϵ��__________________________________________________________________________________��
2P��g��+Q��s����Ӧ�����±�����Ͷ�ϣ���Ӧ�ﵽƽ��״̬�������ϵѹǿ���ߣ������÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��ϵ��__________________________________________________________________________________��| �� �� | M | N | P | Q |
| ��ʼͶ��/mol | 2 | 1 | 2 | 0 |
 xC��g�� ��H ��:
xC��g�� ��H ��:

 2C��g��,���� 2 s���룩����C ��Ũ��Ϊ 0.6 mol��L��1 ���� ������ A ��ʾ�ķ�Ӧ��ƽ������ �� 2 s ʱ���� B ��Ũ��Ϊ ��
2C��g��,���� 2 s���룩����C ��Ũ��Ϊ 0.6 mol��L��1 ���� ������ A ��ʾ�ķ�Ӧ��ƽ������ �� 2 s ʱ���� B ��Ũ��Ϊ �� CO2(g)��H2(g)
CO2(g)��H2(g)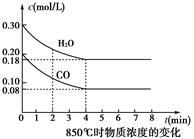



 �Ļ�ѧƽ�ⳣ
�Ļ�ѧƽ�ⳣ ��
��