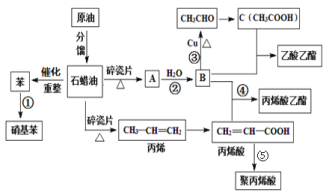
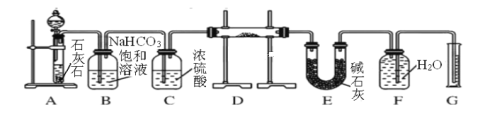
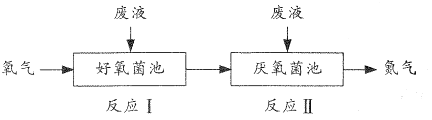
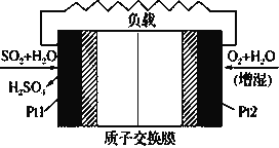
��Ŀ����
����Ŀ����װ�ķ��ݻ��ͷų��ж��ļ�ȩ���塣��-Ferrozine������ȩ��HCHO����ԭ�����£���ԭ�����������������ȩ�ķ�Ӧ��������˵����ȷ����

A.����������HCHO��Fe3+�����ʵ���֮��Ϊ4:1
B.30gHCHO������ʱ�������ϵ�·��ͨ��2mol����
C.��������ĵ缫��ӦʽΪAg2O+2H++2e-=2Ag+H2O
D.����������ͬ����ȩŨ��Խ��������ɫ�������Һ�������ԽС
���𰸡�C
��������
HCHO��CΪ0�ۣ�����-Ferrozine������ȩ��HCHO����ԭ����֪���ü������漰�Ļ�ѧ��ӦΪ2Ag2O��HCHO=4Ag��CO2����H2O��Ag��Fe3��=Ag����Fe2����������HCHO�ڸ���ʧ���ӷ���������Ӧ����CO2���缫��ӦʽΪHCHO��4e����H2O=CO2����4H����������Ag2O�õ��ӷ�����ԭ��Ӧ����Ag���缫��ӦʽΪ2Ag2O��4e����4H��=4Ag��2H2O��
A��ɷ�Ӧ�ķ���ʽ�ɵ�HCHO��4Ag��4Fe2������1 mol HCHO��ȫ��Ӧ��������������4 mol Ag������4 mol Fe3����HCHO��Fe3+�����ʵ���֮��Ϊ1:4����A����
B�30gHCHO�����ʵ���Ϊ1 mol����HCHO�ڸ�������������Ӧ����CO2����������1 mol HCHO�������ϵ�·��ͨ��4 mol���ӣ���B����
C�������Ag2O�õ��ӷ�����ԭ��Ӧ����Ag���缫��ӦʽΪ2Ag2O��4e����4H��=4Ag��2H2O����C��ȷ��
D��������Ϣ��֪�����������ɫ���ʵ�Ũ�ȳ����ȣ����ݷ�Ӧʽ�����Ƴ���������ȩ��Ũ�ȳ����ȣ���D����
��ѡC��