��Ŀ����
����Ŀ��ij��ʵ������480mL��0.5mol/L��ϡH2SO4��Һ��ijͬѧ��98%��ŨH2SO4(��=1.84g/cm3)�������ƣ���ش��������⣺
(1)ʵ����Ҫ�IJ������������ձ�����Ͳ��������������____________��
(2)���㣺98%��ŨH2SO4(��=1.84g/cm3)�����ʵ���Ũ��Ϊ_____________�����Ʊ���ʵ����Ҫ��ϡ����������Ͳ��ȡ����98%��ŨH2SO4_______ mL��
(3)���ƹ��̣�������Ͳ��ȡ�����Ũ���ᡣ
�ڽ�Ũ���Ỻ��ע��ʢ����������ˮ���ձ��У��ӱ߽��衣
���ò������������ձ��е���Һת�Ƶ��Ѿ���©�ĺ��ʹ�������ƿ�С�
��ϴ���ձ��Ͳ�����2��3�Σ�ϴ��ҺҲע������ƿ�У�����ҡ������ƿ��ʹ��Һ��Ͼ��ȡ�
��������ƿ�м�������ˮ���ھ���̶�1��2cmʱ�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
�Ǻ�ƿ�����������µߵ���ҡ�ȡ�
�߽����ƺõ���Һת�����Լ�ƿ�д��á�
����������Һ�IJ������ȱʧ��ȱ�ٵIJ�����__________________��Ӧ���ڲ���_____֮ǰ����(���������)��
(4)�ں�������д���и����������������ҺŨ�ȵ�Ӱ��(ѡ����ƫ��������ƫ����������Ӱ����).
����ȡŨ����������Ͳ������ˮ_________��
�ڶ���ʱ������Һ��_________��
������Ͳ��ȡŨ����ʱ����Һ��___________��
���𰸡���ͷ�ιܡ�500mL����ƿ 18.4mol/L 13.6 ���ձ��е���Һ��ȴ������ �� ƫ�� ƫ�� ƫ��
��������
(1)����һ�����ʵ���Ũ�ȵ���Һ��һ��Ҫʹ��һ����������ƿ����ʵ����û��480mL������ƿ������ѡ��500mL������ƿ������ʱ��ʹ�ý�ͷ�ιܡ�
(2)����98%��ŨH2SO4(��=1.84g/cm3)�����ʵ���Ũ��ʱ��ʹ�ù�ʽ![]() ���м��㣻���Ʊ���ʵ����Ҫ��ϡ���ᣬ������ϡ�Ͷ��ɣ���������98%��ŨH2SO4�������
���м��㣻���Ʊ���ʵ����Ҫ��ϡ���ᣬ������ϡ�Ͷ��ɣ���������98%��ŨH2SO4�������
(3)���ƹ���ʱ���������Ʋ��裺���㡢�������ܽ⡢��ȴת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȣ��������������ҳ���ȱʧ�IJ��輰����λ�á�
(4)�Ӳ���������ȡ��Ũ��������������Һ�����Ӱ�죬��������������ҺŨ�ȵ�Ӱ�졣
(1)ʵ����Ҫ�IJ������������ձ�����Ͳ��������������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
(2)98%��ŨH2SO4(��=1.84g/cm3)�����ʵ���Ũ��Ϊ![]() mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.5mol/L����ã�x��13.6���ʴ�Ϊ��18.4mol/L��13.6��
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.5mol/L����ã�x��13.6���ʴ�Ϊ��18.4mol/L��13.6��
(3)��������һ�����ʵ���Ũ�ȵ���Һ���裺���㡢�������ܽ⡢��ȴת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȡ�ȱ�ٵIJ����ǽ��ձ��е���Һ��ȴ�����£��ò���Ӧ���ڲ����֮ǰ���У��ʴ�Ϊ�����ձ��е���Һ��ȴ�����£��ۣ�
(4)����ȡŨ����������Ͳ������ˮ���൱��ϡ����Ũ���ᣬ������ȡ��Ũ��������������ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ڶ���ʱ������Һ�棬������Һ�����ƫС�����Ƶ���ҺŨ��ƫ�ߣ�
������Ͳ��ȡŨ����ʱ����Һ�棬������ȡ��Ũ��������ƫ����������ʵ���ƫ�����Ƶ���ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ͣ�ƫ�ߣ�ƫ�ߡ�

����Ŀ��NF3�������������ڳ��³�ѹ������ɫ����ζ�����壬�����ӹ�ҵ��һ�������ĵ�����ʴ�����塣�ش��������⣺
��1��NF3�ĵ���ʽΪ______��NԪ�صĻ��ϼ�Ϊ______��
��2��F2��NH3ֱ�ӷ�Ӧ����NF3�Ļ�ѧ����ʽΪ______��
��3��ʵ����ģ�ҵ�����õ������NH4HF2��NH4FHF������ȡNF3������Ϊ��NiΪ�������ϵĺϽ��ں����������������������������ķ�Ӧ��������Ϊ̼�ظ֣�����Һ�ɻ��������á�
�ٵ��ʱNF3��______�����ɣ�����������������______���ѧʽ����
�ڵ����Һ����Ni����Fe��Cu�ĵ��ʼ�NH4HF2�ȣ��ɾ��������̽��л��������ã�
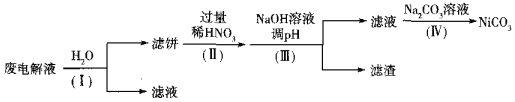
��֪��ʵ�������£����ֽ������ӿ�ʼ�����������ȫ��pH���±�
�������� | Ni2+ | Fe2+ | Cu2+ | Fe3+ |
��ʼ����ʱ��pH | 7.2 | 7.0 | 4.7 | 1.9 |
������ȫʱ��pH | 9.2 | 9.0 | 6.7 | 3.2 |
����I��Ŀ����______��������˱���Ni������������ӷ���ʽΪ______��HNO3�Ļ�ԭ����ΪNO������������pHʱ��������pHӦ���Ƶķ�Χ��______��