��Ŀ����
����Ŀ�������ѣ�DME������Ϊ��21���͵����ȼ�������ɺϳ����Ʊ������ѵ���Ҫԭ�����£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H 1=��90.7 kJ��mol-1 K1
CH3OH(g) ��H 1=��90.7 kJ��mol-1 K1
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H 2=��23.5 kJ��mol-1 K2
CH3OCH3(g)+H2O(g) ��H 2=��23.5 kJ��mol-1 K2
��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H 3=��41.2kJ��mol-1 K3
CO2(g)+H2(g) ��H 3=��41.2kJ��mol-1 K3
�ش��������⣺
��1����Ӧ3H2(g)��3CO(g)![]() CH3OCH3(g)��CO2(g)����H��____kJ��mol-1���÷�Ӧ��ƽ�ⳣ��K=____����K1��K2��K3��ʾ��
CH3OCH3(g)��CO2(g)����H��____kJ��mol-1���÷�Ӧ��ƽ�ⳣ��K=____����K1��K2��K3��ʾ��
��2�����д�ʩ�У������CH3OCH3���ʵ���____��
A��ʹ�ù�����CO B�������¶� C������ѹǿ
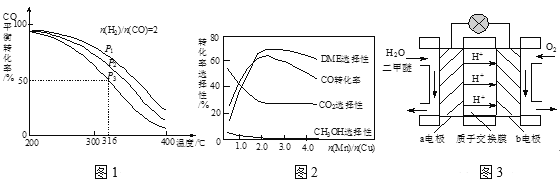
��3�����ϳ�����n(H2)/n(CO)=2ͨ��1 L�ķ�Ӧ���У�һ�������·�����Ӧ��4H2(g)+2CO(g)![]() CH3OCH3(g)+H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ1��ʾ������˵����ȷ����____��
CH3OCH3(g)+H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ1��ʾ������˵����ȷ����____��
A����H <0
B��P1>P2>P3:
C������P3��316��ʱ����ʼʱn(H2)/n(CO)=3����ﵽƽ��ʱ��COת����С��50��
��4������һ�����͵Ĵ�������Ҫ�ɷ���Cu-Mn�ĺϽ𣩣�����CO��H2�Ʊ������ѡ��۲�ͼ2�ش����⡣������n(Mn)/n(Cu)ԼΪ____ʱ�������ڶ����ѵĺϳɡ�
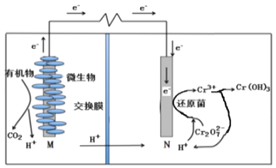
��5��ͼ3Ϊ��ɫ��Դ��������ȼ�ϵ�����Ĺ���ԭ��ʾ��ͼ��a�缫�ĵ缫��ӦʽΪ_____��
���𰸡�-246.1 K12��K2��K3 AC AB 2.0(2-3֮�伴��) CH3OCH3+3H2O-12e-=2CO2��+12H+
��������
(1)�����Ȼ�ѧ����ʽ��˹���ɼ���õ������ݻ�ѧƽ�ⳣ�������Ϸ�Ӧ��ѧ����ʽ��дƽ�ⳣ�������ƽ�ⳣ������ʽ����õ�ƽ�ⳣ����ϵ��
(2)���CH3OCH3���ʣ���ƽ�������ƶ�������Ӱ��ƽ������ط�����
(4)A�������¶ȶ�CO��ת���ʵ�Ӱ�������
B���÷�Ӧ������Ϊ�����С�ķ�����ѹǿ��COת���ʵ�Ӱ�������
C������P3��316��ʱ����ʼʱ![]() =3��������������������
=3��������������������
(5)����ͼ�����ɶ����ѵ����ֵ������
(6)���������£�������ʧ�������ɶ�����̼��
(1)��֪��CO(g)+2H2(g)![]() CH3OH( g)��H1=-90.7kJmol-1��K1=
CH3OH( g)��H1=-90.7kJmol-1��K1=![]() ����2CH30H(g)
����2CH30H(g)![]() CH30CH3(g)+H2O(g)��H2=-23.5kJmol-1��K2=
CH30CH3(g)+H2O(g)��H2=-23.5kJmol-1��K2=![]() ����CO(g)+H2O(g)
����CO(g)+H2O(g)![]() CO2(g)+H2(g)��H3=-41.2kJmol-1��K3=
CO2(g)+H2(g)��H3=-41.2kJmol-1��K3=![]() �����ݸ�˹���ɣ�����2+��+�۵�3CO(g)+3H2(g)
�����ݸ�˹���ɣ�����2+��+�۵�3CO(g)+3H2(g)![]() CH3OCH3(g)+CO2(g)��H=-246.1kJmol-1��ƽ�ⳣ��K=
CH3OCH3(g)+CO2(g)��H=-246.1kJmol-1��ƽ�ⳣ��K=![]() = K12��K2��K3��
= K12��K2��K3��
(2)A������Ӧ���Ũ��ƽ�����ƣ�����ʹ�ù�����CO�������CH3OCH3���ʣ���A��ȷ��
B���÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����ƣ���CH3OCH3���ʻή�ͣ���B����
C���÷�Ӧ������Ϊ�����С�ķ�����������ѹǿƽ�����ƣ������CH3OCH3���ʣ���C��ȷ��
�ʴ�ΪAC��
(4)A����ͼ��֪���¶����ߣ�CO��ת���ʽ��ͣ�˵�������¶�ƽ�����ƣ���������Ϊ���ȷ�Ӧ������H��0����A��ȷ��
B���÷�Ӧ������Ϊ�����С�ķ�������ѹǿCO��ת������������P1��P2��P3����B��ȷ��
C������P3��316��ʱ����ʼʱ![]() =3������������������������������Ũ�ȣ�ƽ�����ƣ�CO��ת������������COת���ʴ���50%����C����
=3������������������������������Ũ�ȣ�ƽ�����ƣ�CO��ת������������COת���ʴ���50%����C����
�ʴ�ΪAB��
(5)��ͼ��֪��������![]() ԼΪ2ʱ��CO��ת����������ɶ����ѵ���ࣻ
ԼΪ2ʱ��CO��ת����������ɶ����ѵ���ࣻ
(6)���������£��������ڸ���ʧ�������ɶ�����̼����缫��ӦʽΪ��CH3OCH3-12e-+3H2O=2CO2��+12H+��

����Ŀ����֪��Ӧ2H2(g)+CO(g)![]() CH3OH(g)��ƽ�ⳣ�����±���������ͬ�����ʵ���Ͷ�ϣ�
CH3OH(g)��ƽ�ⳣ�����±���������ͬ�����ʵ���Ͷ�ϣ�
���CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ�����д�С�Ƚ���ȷ����
ƽ�ⳣ�� | �¶�/�� | ||
500 | 700 | 800 | |
K | 2.50 | 0.34 | 0.15 |
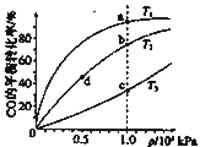
A.ƽ�᳣����K(a)��K(c)��K(b)=K(d)
B.����Ӧ���ʣ�v(a)��v(c)��v(b)=v(d)
C.�ﵽƽ������ʱ�䣺t(a)=t(c)��t(b)��t(d)
D.ƽ����Է���������M(a)=M(c)��M(b)��M(d)