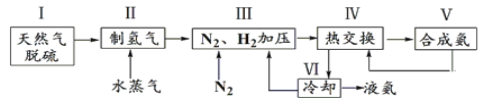
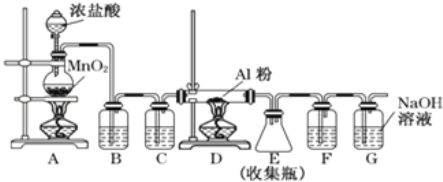
��Ŀ����
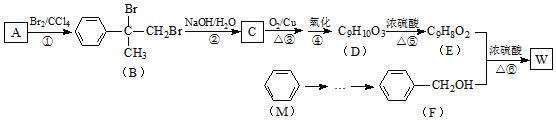
����Ŀ��A����Ȼ������Ҫ�ɷ֣���AΪԭ����һ�������¿ɻ���л���B��C��D��E��F�����ת����ϵ��ͼ����֪��B�ڱ�״���µ��ܶ�Ϊ1.06g��L-1��C�ܷ���������Ӧ��FΪ��Ũ����ζ����������ˮ����״Һ�塣
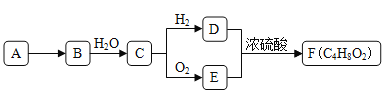
��ش�
(1)�л���D�к��еĹ�����������__________________��
(2) D+E��F�ķ�Ӧ������_________________________��
(3)�л���A�ڸ�����ת��ΪB�Ļ�ѧ����ʽ��_________________��
(4)����˵����ȷ����___��
A.�л���E������Ʒ�Ӧ��ˮ������Ʒ�ӦҪ����
B.�л���D��E��F���ñ���Na2CO3��Һ����
C.ʵ�����Ʊ�Fʱ��Ũ������Ҫ����������
D.�л���C�ܱ����Ƽ���������ͭ����Һ������KMnO4��Һ����
���𰸡� �Ȼ� ������Ӧ 2CH4![]() C2H2+3H2 BD
C2H2+3H2 BD
��������A����Ȼ������Ҫ�ɷ֣�A�Ǽ��顣��AΪԭ����һ�������¿ɻ���л���B��C��D��E��F����֪��B�ڱ�״���µ��ܶ�Ϊ1.16g��L-1��B����Է���������1.16��22.4��26������B����Ȳ��C�ܷ���������Ӧ��˵����Ȳ��ˮ�ӳ�������ȩ����ȩ�����������ᣬE�����ᡣ��ȩ����ԭ�����Ҵ���D���Ҵ����Ҵ���������������FΪ��Ũ����ζ��������������������ˮ����״Һ�塣(1)�л���D���Ҵ������к��еĹ������������ǻ���(2) D+E��F�ķ�Ӧ������������Ӧ��(3)�л���A�ڸ�����ת��ΪB������ԭ���غ��֪�����������ɣ���Ӧ�Ļ�ѧ����ʽ��2CH4![]() C2H2+3H2��(4)A.�Ҵ����ǵ���ʣ��л���D������Ʒ�Ӧ��ˮ������Ʒ�ӦҪ����A����B.�Ҵ���ˮ���ܣ������̼���Ʒ�Ӧ����CO2����������������ˮ���Ҵ��л���D��E��F���ñ���Na2CO3��Һ����B��ȷ��C.ʵ�����Ʊ�Fʱ��Ũ������Ҫ���������ˮ�����ã�C����D.�л���C����ȩ���ܱ����Ƽ���������ͭ����Һ������KMnO4��Һ������D��ȷ����ѡBD��
C2H2+3H2��(4)A.�Ҵ����ǵ���ʣ��л���D������Ʒ�Ӧ��ˮ������Ʒ�ӦҪ����A����B.�Ҵ���ˮ���ܣ������̼���Ʒ�Ӧ����CO2����������������ˮ���Ҵ��л���D��E��F���ñ���Na2CO3��Һ����B��ȷ��C.ʵ�����Ʊ�Fʱ��Ũ������Ҫ���������ˮ�����ã�C����D.�л���C����ȩ���ܱ����Ƽ���������ͭ����Һ������KMnO4��Һ������D��ȷ����ѡBD��