��Ŀ����
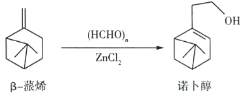
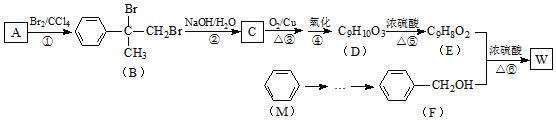
����Ŀ���л���W������������߷��Ӳ��Ϻϳɵ��м���ȣ��Ʊ�W��һ�ֺϳ�·����ͼ��
��֪��![]()
![]()
![]()
![]()
![]()
��ش��������⣺
![]() �Ļ�ѧ������______��
�Ļ�ѧ������______��![]() �ķ�Ӧ������______
�ķ�Ӧ������______
![]() �к��еĹ�������_______
�к��еĹ�������_______![]() д����
д����![]() ��D�ۺ����ɸ߷��ӻ�����Ľṹ��ʽΪ______
��D�ۺ����ɸ߷��ӻ�����Ľṹ��ʽΪ______
![]() ��Ӧ
��Ӧ![]() �Ļ�ѧ����ʽ��______
�Ļ�ѧ����ʽ��______
![]() ��Ӧ
��Ӧ![]() �Ļ�ѧ����ʽ��______
�Ļ�ѧ����ʽ��______
![]() ���㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ______
���㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ______
![]() �����л���W�������ϳ�·�ߣ������MΪ��ʼԭ���Ʊ�F�ĺϳ�·��
�����л���W�������ϳ�·�ߣ������MΪ��ʼԭ���Ʊ�F�ĺϳ�·��![]() ���Լ���ѡ
���Լ���ѡ![]() ʾ����CH3CH2OH
ʾ����CH3CH2OH![]() CH2=CH2
CH2=CH2![]() BrCH2CH2Br]______
BrCH2CH2Br]______
���𰸡����״� ˮ�ⷴӦ��ȡ����Ӧ �ǻ����Ȼ�  2
2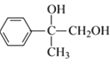 +O2
+O2![]() 2
2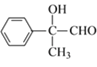 +2H2O
+2H2O ![]() +
+![]()
![]()
![]() +H2O
+H2O 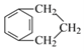
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
�����̿�֪����Ϊ�ӳɷ�Ӧ����B�Ľṹ��֪AΪ![]() ��B�к�-Br����Ϊˮ�ⷴӦ����CΪ
��B�к�-Br����Ϊˮ�ⷴӦ����CΪ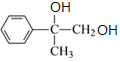 ��C������������������D��DΪ
��C������������������D��DΪ ��D��Ũ�������ʱ��ˮ����E��EΪ
��D��Ũ�������ʱ��ˮ����E��EΪ![]() ��E��F����������Ӧ����WΪ
��E��F����������Ӧ����WΪ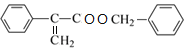 ��
��
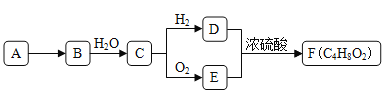
��6������Ϣ![]() ��֪�����ɱ��Ʊ��ױ����ױ��������������·�������ȡ������ˮ�����ɴ����Դ������
��֪�����ɱ��Ʊ��ױ����ױ��������������·�������ȡ������ˮ�����ɴ����Դ������
�����̿�֪��![]() Ϊ�ӳɷ�Ӧ����B�Ľṹ��֪AΪ
Ϊ�ӳɷ�Ӧ����B�Ľṹ��֪AΪ![]() ��B�к�
��B�к�![]() ��
��![]() Ϊˮ�ⷴӦ����CΪ
Ϊˮ�ⷴӦ����CΪ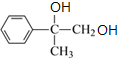 ��C������������������D��DΪ
��C������������������D��DΪ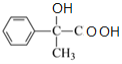 ��D��Ũ�������ʱ��ˮ����E��EΪ
��D��Ũ�������ʱ��ˮ����E��EΪ![]() ��E��F����������Ӧ����WΪ
��E��F����������Ӧ����WΪ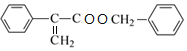 ��
��
![]() Ϊ
Ϊ![]() �������DZ��״���
�������DZ��״���![]() �ķ�Ӧ������ˮ�ⷴӦ��ȡ����Ӧ��
�ķ�Ӧ������ˮ�ⷴӦ��ȡ����Ӧ��
![]() Ϊ
Ϊ ��D�к��еĹ��������ǻ����Ȼ���D�������۷�Ӧ����
��D�к��еĹ��������ǻ����Ȼ���D�������۷�Ӧ����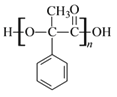 ����ѧ��ӦΪ
����ѧ��ӦΪ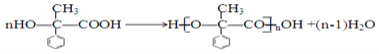 ��
��
![]() ��Ӧ
��Ӧ![]() Ϊ���Ĵ���������ѧ����ʽΪ2
Ϊ���Ĵ���������ѧ����ʽΪ2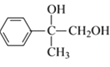 +O2
+O2![]() 2
2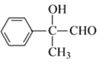 +2H2O���ʴ�Ϊ��2
+2H2O���ʴ�Ϊ��2 +O2
+O2![]() 2
2 +2H2O��
+2H2O��
![]() ��Ӧ
��Ӧ![]() ΪE��F������������Ӧ���仯ѧ����ʽ��
ΪE��F������������Ӧ���仯ѧ����ʽ��![]() ��
��
![]() Ϊ
Ϊ![]() �����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ
�����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ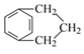 ��
��
![]() ����Ϣ
����Ϣ![]() ��֪�����ɱ��Ʊ��ױ����ױ��������������·�������ȡ������ˮ�⼴�ɣ�����ͼΪ
��֪�����ɱ��Ʊ��ױ����ױ��������������·�������ȡ������ˮ�⼴�ɣ�����ͼΪ![]()
![]()
![]()
![]()
![]()
![]()
![]() ��
��