��Ŀ����
����Ŀ�������ķ�չ�봴������ֹ�����ҹ�����ר�Һ�°�ĸ����·����������ά�Ĵ����������գ�ʹ��������ijɱ����Խ��͡��������̿ɼ�Ҫ��ʾ��ͼ��

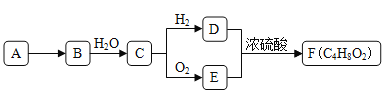
(1)���������ͨ��CO2�Ͱ�����Ӧ��ͨ��___________���ѧʽ����
(2)�������з�����Ӧ�Ļ�ѧ����ʽ___________��
(3)��ĸҺ��ͨ�������ټ���ϸСʳ�ο�������ȴ�����ĸ���Ʒ��___________������ϸСʳ�ο�����������___________��
(4)�ȼҵ��ԭ�ϱ���ʳ��ˮ�к���һ������笠����ӣ��ڵ��ʱ���������ʼ����ȶ������Ȼ�������������ը�����Ȼ�����ˮ�⣬��ˮ��������������ɰ����⣬��һ����Ϊ___________���ѧʽ����Ϊ��ȥ����ʳ��ˮ�е�笠����ӣ����ڼ���������ͨ����������Ӧ���ɵ������÷�Ӧ�����ӷ���ʽΪ___________��
(5)�����Ƽ�ƵõĴ����к���̼���������ʡ����ó������ⶨ����Ĵ��ȣ�ȡm1g��Ʒ����ˮ�ܽ⣬�ټӹ�����CaCl2��Һ����ַ�Ӧ���ˡ�ϴ�ӡ������Ƶó���������Ϊm2g�������Ʒ��̼���ƵĴ���Ϊ��___________��
���𰸡�NH3 NH3��CO2��NaCl��H2O=NaHCO3����NH4Cl NH4Cl ����Cl��Ũ�ȣ�ʹNH4Cl�������� HClO 3Cl2��2![]() ��8OH��=N2��6Cl����8H2O
��8OH��=N2��6Cl����8H2O ![]() %
%
��������
�ϳɰ�������ԭ���������ø�����CO2��������ʳ��ˮ����ͨ��NH3����ͨ��CO2��������ӦNH3+NaCl+H2O+CO2==NaHCO3��+NH4Cl�����˺����ó���������¯�ڼ��ȣ�������Ӧ2NaHCO3![]() Na2CO3+CO2��+H2O�����ɵ�CO2��ͨ������أ�ѭ��ʹ�ã�ĸҺ��ͨ�������ټ���ϸСʳ�ο�����NH4Cl�ᾧ������ʣ����Һѭ��ʹ�á��ƵõĴ����к���̼���������ʣ�����ˮ�����CaCl2��Һ��ͨ���ⶨ��������������ȷ��Na2CO3�����������ȡ�
Na2CO3+CO2��+H2O�����ɵ�CO2��ͨ������أ�ѭ��ʹ�ã�ĸҺ��ͨ�������ټ���ϸСʳ�ο�����NH4Cl�ᾧ������ʣ����Һѭ��ʹ�á��ƵõĴ����к���̼���������ʣ�����ˮ�����CaCl2��Һ��ͨ���ⶨ��������������ȷ��Na2CO3�����������ȡ�
(1)��NaCl��Һ�У�CO2���ܽ�Ȳ������ڼ�����Һ�У�CO2���ܽ�Ȼ�����ܶ࣬�������������ͨ��CO2�Ͱ�����Ӧ��ͨ��NH3����Ϊ��NH3��
(2)���������֪���������з�����Ӧ�Ļ�ѧ����ʽΪNH3��CO2��NaCl��H2O=NaHCO3����NH4Cl����Ϊ��NH3��CO2��NaCl��H2O=NaHCO3����NH4Cl��
(3)��ĸҺ��ͨ�������ټ���ϸСʳ�ο�������ʱNH4Cl�����������࣬�ܽ������٣���ȴ�����ĸ���Ʒ��NH4Cl������ϸСʳ�ο���������������Cl��Ũ�ȣ�ʹNH4Cl������������Ϊ��NH4Cl������Cl��Ũ�ȣ�ʹNH4Cl����������
(4)��NCl3�У�N��-3�ۣ�Cl��+1�ۣ����Ȼ�����ˮ�⣬��ˮ��������������ɰ����⣬��һ����ΪHClO��Ϊ��ȥ����ʳ��ˮ�е�笠����ӣ����ڼ���������ͨ����������Ӧ���ɵ���(N��-3�����ߵ�0��)����Cl�ļ�̬Ӧ���ͣ�����0�۽���Ϊ-1�ۣ��Ӷ�����Cl-���÷�Ӧ�����ӷ���ʽΪ3Cl2��2![]() ��8OH��=N2��6Cl����8H2O����Ϊ��HClO��3Cl2��2
��8OH��=N2��6Cl����8H2O������HClO��3Cl2��2![]() ��8OH��=N2��6Cl����8H2O��
��8OH��=N2��6Cl����8H2O��
(5)�ɷ�ӦNa2CO3+CaCl2==CaCO3��+2NaCl�������m(Na2CO3)=![]() =
= ![]() g������Ʒ��̼���ƵĴ���Ϊ��
g������Ʒ��̼���ƵĴ���Ϊ��![]() =
=![]() %������
%������![]() %��
%��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�����Ŀ���к͵ζ��Ǹ��л�ѧ��Ҫ�Ķ���ʵ�顣��ش��������⣺
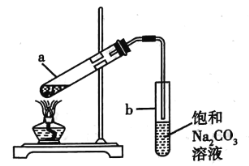
��1���ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������������ɲⶨ���ƫ�ߵ���________(��ѡ����ĸ)��
A.�ζ��յ����ʱ�����ӵζ��̶ܿ�
B.ʢװNaOH��Һ����ƿ������ˮϴ����δ��NaOH��Һ��ϴ
C.��ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D.�ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
��2��ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.02 mol��L��1�ĸ��������Һ�ζ���������Ӧ�����ӷ���ʽΪ��_________________
��3���ζ��������£�
�ζ����� | ����Һ�����mL�� | ��KMnO4��Һ�����mL�� | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 25.00 | 0.50 | 20.40 |
�ڶ��� | 25.00 | 3.00 | 23.40 |
������ | 25.00 | 4.00 | 24.10 |
�ٵζ�ʱ���÷�Ӧ���ʿ�ʼʮ�ֻ�����һ��ʱ���ͻȻ�ӿ죬������Ϊ_________�������ӣ��Ը÷�Ӧ���д����ã�KMnO4��ҺӦװ��_____(��ᡱ�)ʽ�ζ����У��ζ��յ��������_____________________��
�ڸò�����Һ�����ʵ���Ũ��Ϊ____________��