��Ŀ����
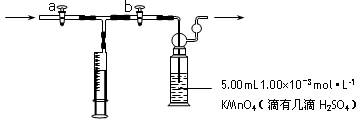
��15�֣�ijУ�о���ѧϰС���������ͼ��ʾװ�ý����й�ʵ�飬ʵ��ʱ����Һ©����A��μ�����ƿB�У���ͼ������̨����������ȥ��Ҫ����д���Լ��ľ��ѧʽ��

��1����AΪŨ���ᣬBΪ�������ڽ���Ԫ�صĵ��ʣ�
���ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ��
�۲쵽C����Һ��ɫ����B�� �������ж�B������ ��
��ʹC�Թ���Һ�ָ�ԭ������ɫ���ɲ�ȡ�IJ���Ϊ ��
��2����BΪ��״����ʯ��CΪ����Na2CO3��Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ���C�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3����B����ʯ�ң�ʵ���й۲쵽C��Һ�����γɳ�������������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ��C����ʢ�з�ˮ���ձ��У���ֹƬ�̣��۲쵽�Թܱ��ϳ��ֹ�����������A�� ��C�� �������ǵĻ��Һ������D�ڴ�ʵ���е������� ��
��4�����ô���װ�����ʵ��֤��̼�ᡢ���ᡢ���ӵ�����ǿ������A��B��C�зŵ��Լ��ֱ��� �� �� ��B��C��ʵ������ֱ�ΪB ��C ����ʹ�������ԣ�����C�е���ҺӦ��ϡ��Һ����Ũ��Һ ������Ũ��ϡ��

��1����AΪŨ���ᣬBΪ�������ڽ���Ԫ�صĵ��ʣ�
���ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ��
�۲쵽C����Һ��ɫ����B�� �������ж�B������ ��
��ʹC�Թ���Һ�ָ�ԭ������ɫ���ɲ�ȡ�IJ���Ϊ ��
��2����BΪ��״����ʯ��CΪ����Na2CO3��Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ���C�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3����B����ʯ�ң�ʵ���й۲쵽C��Һ�����γɳ�������������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ��C����ʢ�з�ˮ���ձ��У���ֹƬ�̣��۲쵽�Թܱ��ϳ��ֹ�����������A�� ��C�� �������ǵĻ��Һ������D�ڴ�ʵ���е������� ��
��4�����ô���װ�����ʵ��֤��̼�ᡢ���ᡢ���ӵ�����ǿ������A��B��C�зŵ��Լ��ֱ��� �� �� ��B��C��ʵ������ֱ�ΪB ��C ����ʹ�������ԣ�����C�е���ҺӦ��ϡ��Һ����Ũ��Һ ������Ũ��ϡ��
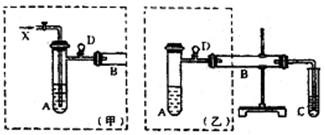
��15�֣���1��B��Mg�������ǵ�������Ԫ��Na��Mg��Al�Т��ڳ���������ˮ��Ӧ�ų�Na���ڳ�������Ũ���ᷴӦ�ų�Al�������Թ�C������ˮԡ
��2��Na2CO3+CO2+H2O=2NaHCO3��
��3��A��ŨNH3��H2O��C��AgNO3��D�������Ƿ�ֹ����
��4��CH3COOH��NaHCO3��C6H5ONa��B�������ݲ�����C����Һ����ǣ�Ũ
��2��Na2CO3+CO2+H2O=2NaHCO3��
��3��A��ŨNH3��H2O��C��AgNO3��D�������Ƿ�ֹ����
��4��CH3COOH��NaHCO3��C6H5ONa��B�������ݲ�����C����Һ����ǣ�Ũ
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
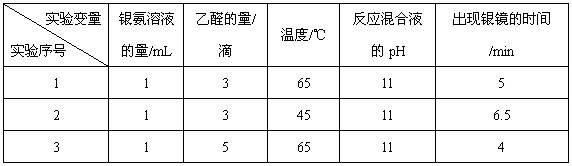

 ����Һ�д���ƽ�⣺[Ag(NH3)2]
����Һ�д���ƽ�⣺[Ag(NH3)2] Ag
Ag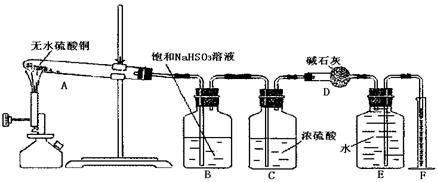
 ��
��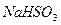 ��Һ����ʱ������
��Һ����ʱ������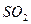 ����
���� �����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
�����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
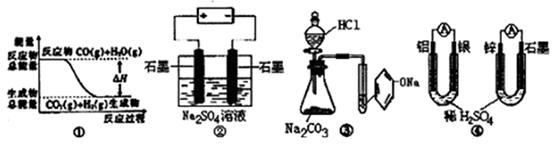
 CO2��g��+H2��g���еġ�H>0
CO2��g��+H2��g���еġ�H>0 ��صĸ���
��صĸ���