��Ŀ����
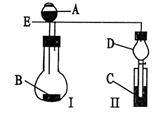
��15�֣���ˮ����ͭ�ڼ����������ܷ����ֽⷴӦ����������ͭ�������������������������ijѧ����ͼ����ͼ��ʾװ����ȷ���û�ѧ��Ӧ�и����ʵļ�����ϵ
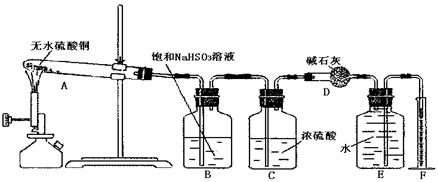
�Իش�
��1�����ȹ����У��Թ�A�з�����ʵ������Ϊ ��
��2��װ��E��F�������� ��
��3����ѧ��ʹ��װ��B�ı����dz�ȥ��������е������������ᴿ����������������ȷ
��Ϊʲô��
��4����ѧ����������װ�ý�һ����������ˮ����ͭ����A�м���ʹ��ֽ⣬�������
�������ƫС����ԭ������������� ��������ţ�
��5����һѧ����4.8g��ˮ����ͭ��ּ���ʹ����ȫ�ֽ������ȷ��ʵ�鷽����ȥ���������еĶ�������������������� �����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
�����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
��6��������ʵ�����ݿ�֪��ˮ����ͭ���ȷֽ�Ļ�ѧ����ʽΪ��
___________________________________________________________________

�Իش�
��1�����ȹ����У��Թ�A�з�����ʵ������Ϊ ��
��2��װ��E��F�������� ��
��3����ѧ��ʹ��װ��B�ı����dz�ȥ��������е������������ᴿ����������������ȷ
��Ϊʲô��
��4����ѧ����������װ�ý�һ����������ˮ����ͭ����A�м���ʹ��ֽ⣬�������
�������ƫС����ԭ������������� ��������ţ�
| A����ˮ����ͭδ��ȫ�ֽ�o*m |
| B��ʵ�����ʱװ��A�в��������� |
C��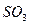 �� ��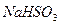 ��Һ����ʱ������ ��Һ����ʱ������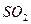 ���� ���� |
| D��������Ͳ�еĶ���ʱ��E�е�Һ�����F�е�Һ�� |
 �����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
�����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol����6��������ʵ�����ݿ�֪��ˮ����ͭ���ȷֽ�Ļ�ѧ����ʽΪ��
___________________________________________________________________
��1����ɫ�����ڣ�2�֣�δд����ɫ�����������ų��������֣�
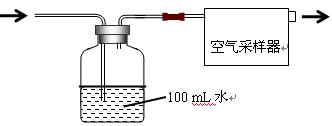
��2���������O2�������2�֣�
��3������ȷ��1�֣���O2�ܱ�NaHSO3��Һ���գ�2�֣����������ɲ����֣�
��4��AD��2�֣� ��5��0.01 0.02 ����2�֣�
��6��3CuSO4 ="==" 3CuO + SO3�� + 2SO2�� + O2�� ��2�֣�
��2���������O2�������2�֣�
��3������ȷ��1�֣���O2�ܱ�NaHSO3��Һ���գ�2�֣����������ɲ����֣�
��4��AD��2�֣� ��5��0.01 0.02 ����2�֣�
��6��3CuSO4 ="==" 3CuO + SO3�� + 2SO2�� + O2�� ��2�֣�
��

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
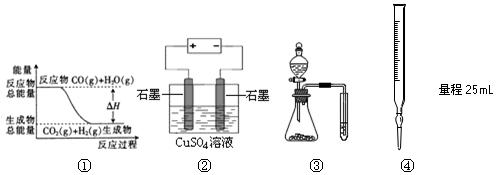
 CO2(g)+H2(g)���еĦ�H����0
CO2(g)+H2(g)���еĦ�H����0 