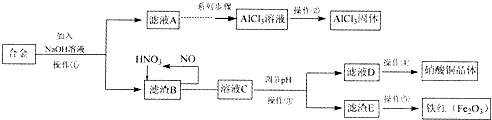
��Ŀ����
5������þ[Mg��ClO3��2]����������������ݼ��ȣ�ʵ������±�飨��Ҫ�ɷ�ΪMgCl2•6H2O������MgSO4��FeCl2�����ʣ��Ʊ�Mg��ClO3��2•6H2O�����������£�
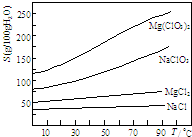
��֪�����ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ��
��1�����������С����������̵����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2����MgO�����������������Ҫ�ɷ�ΪBaSO4��Fe��OH��3��
��3������NaClO3������Һ�������ķ�Ӧ�Ļ�ѧ����ʽΪMgCl2+2NaClO3=Mg��ClO3��2+2NaCl����
��4���ӷ�Ӧ��Ļ�����л��Mg��ClO3��2•6H2O��ʵ�鲽��Ϊ����Ũ�������ȹ��ˡ���ȴ�ᾧ�����ˡ�ϴ�ӣ����й�������Ҫ�IJ����������ձ���©������������
��5����Ʒ��Mg��ClO3��2•6H2O�����IJⶨ�������£�
����1��ȷ����a g��Ʒ�ܽⶨ�ݳ�100mL��Һ��
����2��ȡ10mL������Һ����ƿ�У�����10mLϡ�����15.00mL 1.000mol/L��FeSO4��Һ���ȣ�
����3����ȴ����0.100mol/LK2Cr2O7��Һ�ζ����յ㣮�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7��Һ15.00mL��
�ٲ���2�з�����Ӧ�����ӷ���ʽΪ6Fe2++ClO3-+6H+=6Fe3++Cl-+3H2O��
�ڲ���3�еζ�ǰδ�ñ�Һ��ϴ�ζ��ܣ��ᵼ�½��ƫС���ƫ��ƫС������
��a g��Ʒ������Mg��ClO3��2•6H2O�����ʵ���Ϊ5��10-4mol��
���� ±����Ҫ�ɷ�ΪMgCl2.6H2O������MgSO4��FeCl2�����ʣ�������������������������������Ϊ�����ӣ������Ȼ�����Һ�������ᱵ��������������þ��������Һ��pHΪ4����ʱ�������γ��˳�����������������NaClO3������Һ������ӦΪ��MgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����ˣ��õ�Mg��ClO3��2��Һ�У��������ʵ��ܽ�ȴ�С����Һ��þ���ķ����������ᾧ�����ˡ���ȴ�ᾧ��ȡMg��ClO3��2•6H2O��
��1�����������С�����������Ϊ��������������������Ϊ�����ӣ�
��2������������MgO�����ᷴӦ�����κ�ˮ�����Լ���MgO�������ǵ�����Һ��pH��ʹ����Fe3+�γɳ�����ȫ��ȥ������ʾ��ͼ��֪��������Ҫ�ɷ�ΪBaSO4��Fe��OH��3��
��3������Һ�м���NaClO3������Һ�������ֽⷴӦ����Mg��ClO3��2��NaCl��
��4�����˲��õ�������������̨��©�����ձ����������ȣ��������ڲ��������У�©�����ձ�����������
��5���ٲ���2�У����������£�ClO3-��Fe2+����ΪFe3+����������ԭΪCl-��ͬʱ����H2O����ƽ��д���ӷ���ʽ��
�ڲ���3�����ζ�ǰ���ñ�Һ��ϴ�ζ��ܣ���ϡ��K2Cr2O7��Һ��ʹ����K2Cr2O7��Һ���ƫ��Ӧ�������������ʵ���һ�����ʲⶨClO3-�����ʵ���ƫС��
�۸��ݻ�ѧ����ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O�Լ�Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����Եó���ClO3-��6Fe2+��Cr2O72-��6Fe2+��������K2Cr2O7��Ӧ��Fe2+���ӣ��ټ�����ClO3-��Ӧ��Fe2+���ӣ����ݷ���ʽ����ClO3-�����ʵ������ɵ�Mg��ClO3��2•6H2O�����ʵ�����
��� �⣺��1�����������С�����������Ϊ�������������������ӣ�H2O2��Fe2+�����������·�Ӧ���������Ӻ�ˮ����Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2������������MgO�����ᷴӦ�����κ�ˮ�����Լ���MgO�������ǵ�����Һ��pH��ʹ����Fe3+�γɳ�����ȫ��ȥ������ʾ��ͼ��֪��������Ҫ�ɷ�ΪBaSO4��Fe��OH��3��
�ʴ�Ϊ��BaSO4��Fe��OH��3��
��3������NaClO3������Һ�������ֽⷴӦ����������þ���Ȼ��Ƴ�������Ӧ�ķ���ʽΪ��MgCl2+2NaClO3�TMg��ClO3��2+2NaCl����
�ʴ�Ϊ��MgCl2+2NaClO3=Mg��ClO3��2+2NaCl����
��4�����˵�ԭ���������ǰѲ�����Һ��Ĺ������ʸ�Һ����뿪����һ�ֻ�������ķ���������ʱ��Ҫ������������©������ֽ���̶�����������̨�������õIJ��������н���Һ���ձ��ȣ�������Ҫʹ�õIJ����������ձ�����������©����
�ʴ�Ϊ��©������������
��5���ٸ÷�Ӧ��FeԪ�ػ��ϼ���+2�۱�Ϊ+3�ۡ�ClԪ�ػ��ϼ���+5�۱�Ϊ-1�ۣ���ת�Ƶ���������6������Fe2+�ļ�������6��ClO3-�ļ�������1�����ݵ���غ�֪��H+�ļ�������6������Hԭ���غ�֪��H2O�ļ�������3�����Բ���2�з�����Ӧ�����ӷ���ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
�ʴ�Ϊ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
�ڲ���3�����ζ�ǰ���ñ�Һ��ϴ�ζ��ܣ���ϡ��K2Cr2O7��Һ��ʹ����K2Cr2O7��Һ���ƫ��Ӧ�������������ʵ���һ�����ʲⶨClO3-�����ʵ���ƫС���������ս��ƫС��
�ʴ�Ϊ��ƫС��
�۸��ݻ�ѧ����ʽ��ClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O�Լ�Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����Եó���ClO3-��6Fe2+��Cr2O72-��6Fe2+��Fe2+�������ʵ���Ϊ0.015L��1.00mol/L=0.015mol��K2Cr2O7�����ʵ���Ϊ0.015L��0.100mol/L=0.0015mol������K2Cr2O7��Ӧ��Fe2+����Ϊ0.0015mol��6=0.009mol������ClO3-��Ӧ��Fe2+����Ϊ0.015mol-0.009mol=0.006mol����ClO3-�����ʵ���Ϊ0.006mol��$\frac{1}{6}$=0.001mol����Mg��ClO3��2•6H2O�����ʵ�����5��10-4mol��
�ʴ�Ϊ��5��10-4mol��
���� ���⿼��ʵ���Ʊ��������漰�Բ����ķ������ۡ����ʵķ����ᴿ�����ӷ���ʽ��д�����ʺ����ⶨ��������ԭ��Ӧ�ζ��ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ŀ�Ѷ��еȣ�


pHֵ���ƿɲο���������
| �� �� | ��ʼ����ʱ��pHֵ | ��ȫ����ʱ��pHֵ |
| �������� | 2.7 | 3.7 |
| ���������� | 7.6 | 9.6 |
| ������ͭ | 5.2 | 6.4 |
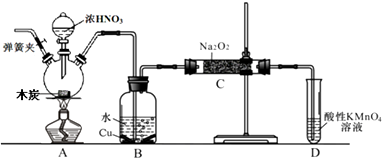
��1��A���ʿ�ѡ��b������ĸ����
a��ϡH2SO4b��ŨH2SO4/��c��ŨFeCl3��Һ d��ŨHNO3
��2�����м�H2O2��Ŀ�Ľ�Fe2+����ΪFe3+��
��3�����м�Cu2��OH��2CO3��Ŀ�����к��������ᣬ������Һ��pH��ʹFe3+ˮ����ȫ�����������ŵ��Dz������µ����ʣ�
��4����������ʱ�����Ļ�ѧ��Ӧ�����ӷ���ʽΪFe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3��+3H+��
��5��V�м�H2SO4����pH=1��Ϊ������Cu2+��ˮ�⣮
��6��V�����IJ���������Ũ������ȴ�ᾧ������
��7��ij����ʦ��Ϊ�������������ӵ�A���ʲ������룬�����Ľ����������ǻ����SO2�������Ⱦ����������������ʵͣ���θĽ�������ϡ�����в���ͨ����������H2O2�������ȣ�
 =BaSO4��
=BaSO4�� =CaCO3����CO
=CaCO3����CO ��2H2O
��2H2O