题目内容
【题目】利用如图装置测定中和热的实验步骤如下:

①用量筒量取50 mL 0.25mol·L-1硫酸倒入小烧杯中,测出硫酸温度;
②用另一量筒量取50 mL 0.55mol·L-1NaOH溶液,并用另一温度计测出其温度;
③将NaOH溶液倒入小烧杯中,设法使之混合均匀,测出混合液最高温度。
回答下列问题:
(1)写出稀硫酸和稀氢氧化钠溶液反应表示中和热的热化学方程式(中和热数值为57.3kJ·mol-1)___________。
(2)倒入NaOH溶液的正确操作是__________(从下列选项中选出)。
A.沿玻璃棒缓慢倒入 B.分三次少量倒入 C.一次迅速倒入
(3)使硫酸与NaOH溶液混合均匀的正确操作是________(从下列选项中选出)。
A.用温度计小心搅拌
B.揭开硬纸片用玻璃棒搅拌
C.轻轻地振荡烧杯
D.用套在温度计上的环形玻璃搅拌棒轻轻地搅动
(4)实验数据如下表:
①请填写下表中的空白:
起始温度t1℃ | 终止温度t2/℃ | 温度差平均值(t2-t1)/℃ | |||
H2SO4 | NaOH | 平均值 | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | _____ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
②近似认为0.55mol·L-1NaOH溶液和0.25mol·L-1硫酸溶液的密度都是1 g·cm-3,中和后生成溶液的比热容c=4.18 J·g-1·℃-1。则中和热ΔH=________(取小数点后一位)。
【答案】![]() H2SO4(aq)+NaOH(aq)=
H2SO4(aq)+NaOH(aq)=![]() Na2SO4(aq)+H2O(l) ΔH=-57.3 kJ·mol-1 C D 3.4 -56.8 kJ·mol-1
Na2SO4(aq)+H2O(l) ΔH=-57.3 kJ·mol-1 C D 3.4 -56.8 kJ·mol-1
【解析】
(1)强酸、强碱的中和热为-57.3kJ·mol-1,是强酸和强碱的稀溶液完全反应生成1mol水放出的热量,则稀硫酸和稀氢氧化钠溶液反应的热化学方程式为![]() H2SO4(aq)+NaOH(aq)=
H2SO4(aq)+NaOH(aq)=![]() Na2SO4(aq)+H2O(l) ΔH=-57.3 kJ·mol-1,故答案为:
Na2SO4(aq)+H2O(l) ΔH=-57.3 kJ·mol-1,故答案为:![]() H2SO4(aq)+NaOH(aq)=
H2SO4(aq)+NaOH(aq)=![]() Na2SO4(aq)+H2O(l) ΔH=-57.3 kJ·mol-1;
Na2SO4(aq)+H2O(l) ΔH=-57.3 kJ·mol-1;
(2)倒入氢氧化钠溶液时,必须一次迅速的倒入,目的是减少热量的散失,不能分几次倒入,否则热量散失,影响测定结果,所以C选项正确,故答案为:C;
(3)使硫酸与氢氧化钠溶液混合均匀的正确操作方法是:用套在温度计上的环形玻璃搅拌棒轻轻的搅动;温度计是测量温度的,不能使用温度计搅拌,A选项错误;也不能轻轻的振荡烧杯,否则可能导致液体溅出或者热量散失,B选项错误,更不能打开硬纸片用玻璃棒搅拌,否则热量散失,影响测定结果,C选项错误;综上,D选项正确,故答案为:D;
(4)①4次温度差分别为:3.4℃、5.1℃、3.3℃、3.5℃,第2组数据无效,温度差平均值为3.4℃,故正确答案为:3.4;
②50mL0.25mol/L硫酸与50mL0.55mol/L NaOH溶液进行中和反应生成水的物质的量为0.05L×0.25mol/L×2=0.025mol,溶液的质量为100mL×1g/mL=100g,温度的变化的值为ΔT=3.4℃,则生成0.025mol水放出的热量Q=cmΔT=4.18J/(g·℃)×100g×3.4℃=1421.2J,即1.4212kJ,所以中和热![]() ,故答案为-56.8 kJ·mol-1。
,故答案为-56.8 kJ·mol-1。

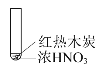
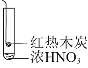
【题目】下述实验中均有红棕色气体产生,对比分析所得结论正确的是( )
① | ② | ③ |
|
|
|
A.由①中的红棕色气体,可推知反应还有氧气产生
B.红棕色气体表明②中木炭与浓硝酸发生了反应
C.由③可说明浓硝酸具有挥发性和强氧化性
D.③的气体产物中检测出CO2,由此说明木炭一定与浓硝酸发生了反应