��Ŀ����
����Ŀ����Ҫ��ش��������⡣
I.����Ԫ�ص�ԭ�ӵ��Ӳ�ṹ���£�A��1s22s22p63s23p63d54s2��B��1s22s22p63s2��C��1s22s22p6��D��1s22s22p63s23p2��E��[Ar]4s1��
��ش�(��Ԫ�ط���)
��1��________Ԫ����ϡ�����塣��δ�ɶԵ���������Ԫ����________��
��2��AԪ��ԭ�ӵĺ�����ӹ���________���˶�״̬��������ߵ��ܼ���________�����ܼ����ţ���
��3��DԪ��ԭ�ӵļ۲�����Ų�ͼ��________��
��4��________Ԫ�صĵ縺�����________Ԫ��ԭ�ӵĵ�һ���������________Ԫ����������ɾ��д����ʵ������
II.Q��R��X��Y��Z����Ԫ�ص�ԭ���������ε�������Z���⣬����ľ�Ϊ����������Ԫ�ء���֪��
��Qԭ��2p�ܼ�����һ���չ����
��Rԭ�Ӻ���L�������Ϊ������
��Xԭ��2p�����ֻ��һ�������෴�ĵ��ӣ�
��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn��
��Zԭ��M�����й��ȫ��������N���ɶԵ��ӣ�ֻ��1��δ�ɶԵ��ӡ���ش��������⣺
��5��Z2���ĺ�������Ų�ʽ��________��XԪ�ػ�̬ԭ�ӵĺ�������Ų�ͼ��____________��
��6��Q��Y�ֱ��γɵ������̬�⻯���У��ȶ��Ը�ǿ����________���ѧʽ����
��7��Q��R��X��Y����Ԫ�صĵ�һ��������ֵ�ɴ�С��˳��Ϊ________����Ԫ�ط������𣩡�
���𰸡�Ne Mn 25 3d ![]() Si Ne Mn 1s22s22p63s23p63d9
Si Ne Mn 1s22s22p63s23p63d9 ![]() CH4 N>O>C>Si
CH4 N>O>C>Si
��������
I��A��������Ų�Ϊ1s22s22p63s23p63d54s2��AΪMnԪ�أ�B�ĺ�������Ų�Ϊ1s22s22p63s2��BΪMgԪ�أ�C�ĺ�������Ų�Ϊ1s22s22p6��ΪNeԪ�أ�D�ĺ�������Ų�Ϊ1s22s22p63s23p2��ΪSiԪ�أ�E�ĺ�������Ų�Ϊ[Ar]4s1��EΪKԪ�ء�
II��Qԭ��2p�ܼ�����һ���չ��������2p��������Ų�Ϊ2p2������ΪCԪ�أ�Rԭ�Ӻ���L�������Ϊ����������ԭ����������Q������L����ӿ���Ϊ5��7��Xԭ��2p�����ֻ��һ�������෴�ĵ�������2p��������Ų�Ϊ2p4��ΪOԪ�أ���RΪNԪ�أ�Yԭ�Ӽ۵���(��Χ����)�Ų�msnmpn����Ϊ����������Ԫ�أ�ԭ����������O����������Χ�����Ų�Ϊ3s23p2ΪSiԪ�أ�Zԭ��M�����й��ȫ��������N���ɶԵ��ӣ�ֻ��1��δ�ɶԵ��ӣ�����۵����Ų�Ϊ3d104s1��ΪCuԪ�ء�
(1)NeԪ��Ϊϡ�����壻���ݸ�ԭ�Ӻ�������Ų�ʽ��֪��֪MnԪ�غ�δ�ɶԵ�����࣬��5����
(2)AΪMn��MnΪ25��Ԫ�أ�ԭ�Ӻ�����25�����ӣ�ÿ�����ӵ��˶�״̬����ͬ��������25���˶�״̬��������ߵ��ܼ�Ϊ3d�ܼ���
(3)DΪSiԪ�أ��۲�����Ų�ʽΪ3s23p2���Ų�ͼΪ![]() ��
��
(4)�ǽ�����Խǿ�縺��Խ������Ԫ���зǽ�������ǿ��ΪSi��һ�������ͬ����Ԫ���������ҵ�һ�����ܳ��������ƣ�ͬ������ϵ��µ�һ�����ܼ�С�����Ե�һ����������ΪNeԪ�أ�MnԪ����������ɾ��д����ʵ������
(5)Z2+ΪCu2+��Cuԭ��ʧȥ����Χ���������γ�ͭ���ӣ�����ͭ���Ӻ�������Ų�ʽΪ1s22s22p63s23p63d9��XΪOԪ�أ����������Ų�ͼΪ��![]() ��
��
(6)C�ķǽ�����ǿ��Si�����������̬�⻯����ȶ�����CH4���ȶ���
(7)ͬ����Ԫ���������ҵ�һ�����ܳ��������ƣ���C��N��OԪ����N�������Ϊ�����״̬����һ�����ܴ�������Ԫ�أ����Ӳ���Խ�࣬��һ������ԽС������SiԪ�ص�һ��������С�����Ե�һ��������ֵ�ɴ�С��˳��ΪN>O>C>Si��

����Ŀ��ʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ���з�Ӧ�Ȳⶨ��

��1��д���÷�Ӧ���Ȼ�ѧ����ʽ[����1mol H2O(l)ʱ�ķ�Ӧ��Ϊ-57.3 kJ��mol-1]________________________��
��2��ȡ50mLNaOH��Һ��30 mL������Һ����ʵ�顣
��ʵ���������±����¶Ȳ�ƽ��ֵΪ_________
�¶� ���� | ��ʼ�¶�t1/�� | ��ֹ�� ��t2/�� | �¶Ȳ� ƽ��ֵ (t2-t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
��������Ϊ0.50 mol��L-1NaOH��Һ��0.50 mol��L-1������Һ���ܶȶ���1.0 g��mL-1���кͺ�������Һ�ı�����c=4.18 J��g-1����-1��������1 mol H2O(l)ʱ�ķ�Ӧ����H=_____���г�����ʽ���ɣ���λΪkJ��mol-1����
������ʵ����ֵ�������-57.3 kJ��mol-1������ƫ���ԭ������_____
a.ʵ��װ�ñ��¡�����Ч����
b.��ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�顣��ش��������⣺
��1������к͵ζ�������Ũ��Ϊ0.1000mol��L��1�ı�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������м�¼��ʵ�����ݣ�
�ζ����� | ����Һ���(mL) | ���������(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.50 | 20.40 |
�ڶ��� | 20.00 | 3.00 | 23.00 |
������ | 20.00 | 4.00 | 24.10 |
�����в�����ɲⶨ���ƫ�ߵ���________(��ѡ����ĸ)
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ��
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ��ʢװ��Һ�ĵζ��ܼ��������ݣ��ζ���������ʧ
�ڸ�NaOH��Һ�����ʵ���Ũ��Ϊ_____________mol/L����С���������λ��Ч���֣�
��2��������ԭ�ζ�����ȡһ�����IJ��ᣨH2C2O4����Һ������ƿ�У���������ϡ���ᣬ�ñ����Ը��������Һ�ζ����ζ�ʱKMnO4��ҺӦװ��______________(��ᡱ�)ʽ�ζ����У��ζ��յ�ʱ�ζ�������_________________________________��
��3�������ζ��D�D�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܡ��ο����е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ����______(��ѡ����ĸ)��
������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | dz�� | �� | ש�� | �� |
Ksp | 1.77��10��10 | 5.35��10��13 | 1.21��10��16 | 1.12��10��12 | 1.0��10��12 |
A��NaClB��NaBrC��NaCND��Na2CrO4
����Ŀ����1���л���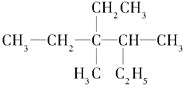 ��ϵͳ������������________________________
��ϵͳ������������________________________
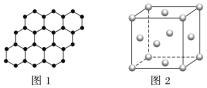
��2��д��4��2�һ�1��ϩ�Ľṹ��ʽ��________________________
��3��ij���Ľṹ��ʽ��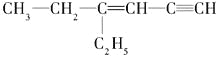 �������б���̼ԭ����Ϊ_____��������ͬһƽ���ϵ�̼ԭ�������Ϊ_________
�������б���̼ԭ����Ϊ_____��������ͬһƽ���ϵ�̼ԭ�������Ϊ_________
��4����������������ͬϵ�����_________
��CH3CH2Cl ��CH2===CHCl ��CH3CH2CH2Cl ��CH2ClCH2Cl ��CH3CH2CH2CH3��CH3CH(CH3)2
A���٢� | B���٢� | C���٢� | D���ݢ� |
��5��0.1 molij����ȼ�գ���ȼ�ղ���ȫ������ʯ�����գ���ʯ����39g�������ķ���ʽΪ________�������ĺ˴Ź���������3���壬��������ܵĽṹ��ʽΪ________��(д������һ�ּ���)
����Ŀ���¶�ΪT1ʱ��������X������Y��1.6mol����10L�����ܱ������У�������ӦX(g)+Y(g)![]() 2Z(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������������˵����ȷ����
2Z(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������������˵����ȷ����
t/min | 2 | 4 | 7 | 9 |
n��Y��/mol | 1.2 | 1.1 | 1.0 | 1.0 |
A. ��Ӧ0��4 min��ƽ�����ʦ�(Z)=0.25 mol/(Lmin)
B. T1ʱ����Ӧ��ƽ�ⳣ��K1��1.2
C. �����������䣬9 min�����������ٳ���1.6 molX��ƽ��������Ӧ�����ƶ����ٴδﵽƽ��ʱX��Ũ�ȼ�С��Y��ת��������
D. �����������䣬���µ�T2�ﵽƽ��ʱ��ƽ�ⳣ��K2=4����˷�Ӧ�ġ�H��0