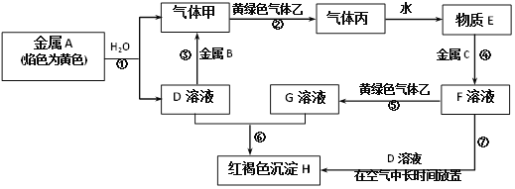
��Ŀ����
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�顣��ش��������⣺
��1������к͵ζ�������Ũ��Ϊ0.1000mol��L��1�ı�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������м�¼��ʵ�����ݣ�
�ζ����� | ����Һ���(mL) | ���������(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.50 | 20.40 |
�ڶ��� | 20.00 | 3.00 | 23.00 |
������ | 20.00 | 4.00 | 24.10 |
�����в�����ɲⶨ���ƫ�ߵ���________(��ѡ����ĸ)
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ��
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ��ʢװ��Һ�ĵζ��ܼ��������ݣ��ζ���������ʧ
�ڸ�NaOH��Һ�����ʵ���Ũ��Ϊ_____________mol/L����С���������λ��Ч���֣�
��2��������ԭ�ζ�����ȡһ�����IJ��ᣨH2C2O4����Һ������ƿ�У���������ϡ���ᣬ�ñ����Ը��������Һ�ζ����ζ�ʱKMnO4��ҺӦװ��______________(��ᡱ�)ʽ�ζ����У��ζ��յ�ʱ�ζ�������_________________________________��
��3�������ζ��D�D�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܡ��ο����е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ����______(��ѡ����ĸ)��
������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | dz�� | �� | ש�� | �� |
Ksp | 1.77��10��10 | 5.35��10��13 | 1.21��10��16 | 1.12��10��12 | 1.0��10��12 |
A��NaClB��NaBrC��NaCND��Na2CrO4
���𰸡�CD 0.1000mol/L �� �������һ�α�Һ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ D
��������
(1)��A���ζ��յ����ʱ�����ӵζ��̶ܿȣ����¶�ȡ�ı�Һ���ƫС���ⶨ���ƫС�����������⣻
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ����Ӱ�����ĵı�Һ��������ԶԽ����Ӱ�죬���������⣻
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ������ϡ�ͱ�Һ�����¶�ȡ�ı�Һ���ƫ�ⶨ���ƫ�ߣ��������⣻
D���ζ�ǰ��ʢװ��Һ�ĵζ��ܼ��������ݣ��ζ���������ʧ�����ȡ�ı�Һ���ƫ�ⶨ���ƫ�������⣻
��������ѡCD��
�ڸ��ݱ���һ�����ı�Һ���Ϊ20.40mL-0.50mL=19.90mL���ڶ������ı�Һ���Ϊ23.00mL-3.00mL=20.00mL�����������ı�Һ���Ϊ24.10mL-4.00mL=20.10mL���������εζ����ĵ�HCl��Һƽ�����Ϊ20.00mL����NaOH��Һ�����ʵ���Ũ��Ϊc=![]() =0.1000mol/L��
=0.1000mol/L��
(2)���Ը��������Һ�����ԣ��Ҿ���ǿ�����ԣ�����װ����ʽ�ζ����У��ζ��յ�ʱ���Ը��������Һ��������Һ��Ϊ�Ϻ�ɫ����������Ϊ���������һ�α�Һ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
(3)����AgNO3ȥ�ζ�NaSCN��Һ����ѡ�õĵζ�ָʾ�������ʵ��ܽ��Ӧ��AgSCN��AgCl��AgBr��AgCN��AgSCN��Ϊͬ���ͳ�����ֻ��AgCl���ܶȻ���AgSCN��AgClͬΪ��ɫ�������������ԣ��������ֳ�����Ӧ���ξ������ʣ�Ag2CrO4��AgSCN�ܶȻ�����Ag2CrO4ΪA2B�ͳ���������Ag2CrO4���ܽ��Ҫ��AgSCN���ܽ��С����Ϊש��ɫ���������ԣ����Կ���ѡNa2CrO4��ָʾ������ѡD��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ������ʵ�������ʵ������������������һ���ǣ� ��
ѡ�� | ʵ����������� | ʵ����� |
A | ��ij������Ʒ�м���Ba(OH)2��Һ���а�ɫ�������� | ����������һ����SO42- |
B | ��KI-������Һ�е�����ˮ����Һ�����ɫ | I-�Ļ�ԭ��ǿ��Cl- |
C | ��Ba(OH)2��8H2O��NH4Cl������С�ձ��л�Ͻ��裬���ִ����ձ���ڸо����� | Ba(OH)2��8H2O��NH4Cl�ķ�Ӧ�����ȷ�Ӧ |
D | ��ij����Һ�м���NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ���� | ������Һ�к���NH4+ |
A.AB.BC.CD.D
����Ŀ����ѧ����������������ء��밴Ҫ�ش��������⣺
��1������ĩ�ڹ��չ��顶�����ǡ��м����С�䳲����ķ����������ú���̼���Ƶ�ˮ��Һ��ϴ��˿������д��̼����ˮ��Һ��ͨ��CO2����Ļ�ѧ����ʽ____����54.8g Na2CO3��NaHCO3 �Ļ����ֳɵ��������ݣ�һ������ˮ������������ᣬ�ռ�������V L����һ��ֱ�Ӽ��������أ���������2.24L����������������ڱ�״���²ⶨ������ԭ����������Na2CO3�����ʵ�����n��Na2CO3����____������V��____��
��2����84������Һ��������ʹ�ù㷺������Ч�ɷ��Ǵ������ơ����ڳ����½�����ͨ��NaOH��Һ�Ƶã��÷�Ӧ�����ӷ���ʽΪ____������2mol��������÷�Ӧ�����ʱת�Ƶĵ�����Ϊ____NA��
��3��С�մ����������θ����࣬�䷴Ӧ�����ӷ���ʽΪ____��
��4����ʯ�ǵر���ʯ����Ҫ�����ҿ��ij�ֳ�ʯ�Ļ�ѧ���KAlSi3O8�����д��������������ʽΪ____��
��5��������(����ʽC6H12O6)�������ϸ����������Դ����֪1mol����1000mmol��ij��쵥��һЩָ����ͼ����ÿ������Ʒ�к������ǵ�����Ϊ____g���뱣����λС������
9 | ����� | 1.6 | |
10 | ��������ø | 161 | U/L |
11 | ���ἡ�ἤø | 56 | U/L |
12 | �������� | 0.52 | mmol/L |
13 | �ܵ��̴� | 4.27 | mmol/L |
14 | ���ܶ�֬�����̴� | 1.57 | mmol/L |
15 | ���ܶ�֬�����̴� | 1.40 | mmol/L |
16 | ������ | 4.94 | mmol/L |
����Ŀ���й���ͳ�Ļ��а�������Ƽ�֪ʶ�����й��������漰��ѧ�仯����
ǧ�����������࣬������ɳʼ���� |
������(CuSO4��5H2O) ��������֮�Ϊͭ |
��ʯ��(CaCO3)�� �������Ϊ�� |
��ɰ(HgS)��֮��ˮ���������ֳɵ�ɰ |
A | B | C | D |
A. A B. B C. C D. D