��Ŀ����
����Ŀ���������ó�Ϊ�����о����ȵ㣬�����͵���з�����Ҫ�IJ��ϡ�
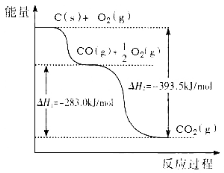
��1��ͨ�����·�Ӧ�Ʊ���������
��Ӧ1��Al2O3(s)��AlCl3(g)��3C(s)===3AlCl(g)��3CO(g)����H1��akJ��mol��1
��Ӧ2��Al2O3(s)��3C(s)===2Al(l)��3CO(g)����H2��bkJ��mol��1
��Ӧ3��3AlCl(g)===2Al(l)��AlCl3(g)����H3
�ٷ�Ӧ3����H3��_______kJ��mol��1��
��950��ʱ���������������Ľ�̿��Cl2��Ӧ���Ƶ�AlCl3���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��2���ڸ��������½��з�Ӧ��2Al(l)��AlCl3(g)![]() 3AlCl(g)��
3AlCl(g)��
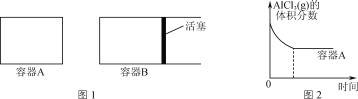
����ͼ1��ʾ�ĵ��ݻ�A��B�ܱ������м���������Al�ۣ��ٷֱ����1 mol AlCl3(g)������ͬ�ĸ����½��з�Ӧ��ͼ2��ʾA�����ڵ�AlCl3(g)���������ʱ��ı仯ͼ����ͼ2�л���B������AlCl3(g)���������ʱ��ı仯���ߡ�__________
��1100��ʱ����2 L�ܱ�������ͨ��3 mol AlCl(g)��������Ӧ��3AlCl(g)=2Al(l)��AlCl3(g)����֪���¶���AlCl(g)��ƽ��ת����Ϊ80%����÷�Ӧ��ƽ�ⳣ��K��________��
�ۼ���3molAlCl(g)���ڲ�ͬѹǿ�·�����Ӧ���¶ȶԲ��ʵ�Ӱ����ͼ3��ʾ���˷�Ӧѡ���¶�Ϊ900���ԭ����_______________��
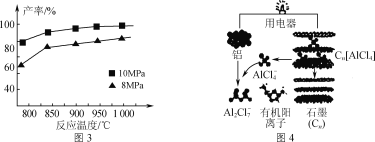
��3�����������Ŀ��ٷŵ������Ӷ��ε�ص�ԭ����ͼ4��ʾ��
�ٸõ�س��ʱ�������ĵ缫��ӦʽΪ_____��
��AlCl3��NaCl�������γ����ڶ������أ����ʱAlCl4-��Al2Cl7-���������ڵ缫���ת�����������Ӳ�����缫��Ӧ��NaCl��������_____��
���𰸡�b��a Al2O3��3C��3Cl2![]() 2AlCl3��3CO
2AlCl3��3CO 
![]() 900��ʱ�������Ѿ��ϸߣ������¶Ȳ������������ܺ����ߣ�����Ч�潵�� 4Al2Cl7-��3e��===Al��7AlCl4- ����AlCl4-��Al2Cl7-��ǿ������
900��ʱ�������Ѿ��ϸߣ������¶Ȳ������������ܺ����ߣ�����Ч�潵�� 4Al2Cl7-��3e��===Al��7AlCl4- ����AlCl4-��Al2Cl7-��ǿ������
��������
��1������֪��Ӧ�٣�Al2O3(s)��AlCl3(g)��3C(s)=3AlCl(g)��3CO(g)����H1��akJ��mol��1
��Ӧ�ڣ�Al2O3(s)��3C(s)=2Al(l)��3CO(g)����H2��bkJ��mol��1
��Ӧ�ۣ�3AlCl(g)=2Al(l)��AlCl3(g)����H3
���ݸ�˹���ɣ��ɢ�-�ٵ÷�Ӧ��3AlCl(g)=2Al(l)��AlCl3(g) ��H3=��H2-��H1= bkJ��mol��1- akJ��mol��1= ��b��a�� kJ��mol��1��
��950��ʱ���������������Ľ�̿��Cl2��Ӧ���Ƶ�AlCl3��ͬʱ��������������һ����̼����Ӧ�Ļ�ѧ����ʽ��Al2O3��3C��3Cl2![]() 2AlCl3��3CO��
2AlCl3��3CO��
��2������ͼ1��ʾ�ĵ��ݻ�A��B�ܱ������м���������Al�ۣ��ٷֱ����1 mol AlCl3(g)������ͬ�ĸ����½��з�Ӧ��ͼ2��ʾA�����ڵ�AlCl3(g)���������ʱ��ı仯ͼ��B����������ɱ䣬����ӦΪ�����������ķ�Ӧ�����ŷ�Ӧ�Ľ������������������������A������Bѹǿ��С����Ӧ���ʼ�����ƽ��������������������Ӧ�����ƶ���ƽ��ʱAlCl3(g)�����������С����ͼ2�л���B������AlCl3(g)���������ʱ��ı仯�������£� ��
��
��1100��ʱ����2 L�ܱ�������ͨ��3 mol AlCl(g)��������Ӧ��3AlCl(g)=2Al(l)��AlCl3(g)����֪���¶���AlCl(g)��ƽ��ת����Ϊ80%����������ʽ�У�
3AlCl(g)=2Al(l)��AlCl3(g)��
��ʼʱŨ�ȣ�mol/L��1.5 0
�ı��Ũ�ȣ�mol/L��1.2 0.4
ƽ��ʱŨ�ȣ�mol/L��0.3 0.4
��÷�Ӧ��ƽ�ⳣ��K��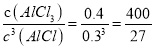 ��
��
�ۼ���3molAlCl(g)���ڲ�ͬѹǿ�·�����Ӧ���¶ȶԲ��ʵ�Ӱ����ͼ3��ʾ����Ӧѡ���¶�Ϊ900���ԭ����900��ʱ�������Ѿ��ϸߣ������¶Ȳ������������ܺ����ߣ�����Ч�潵�ͣ�
��3���ٵ�س��ʱ��������Al2Cl7-�õ��Ӳ�������AlCl4-���缫��ӦʽΪ4Al2Cl7-��3e��===Al��7AlCl4-��
��AlCl3��NaCl�������γ����ڶ������أ����ʱAlCl4-��Al2Cl7-���������ڵ缫���ת����NaCl������������AlCl4-��Al2Cl7-��ǿ�����ԣ��������Ӳ�����缫��Ӧ��

 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д�����Ŀ����Ԫ�صĻ������ڶ�������о�����ҪӦ�á�
��1��Mn2����̬��������Ų�ʽΪ________��SO42-��Sԭ�ӹ�����ӻ�����Ϊ________��
��2�������̵�3�����ӻ�������۵����±���
���� | MnCl2 | MnS | MnO |
�۵� | 650�� | 1610�� | 2800�� |
�ϱ�3�������о�����������________��
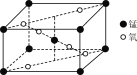
��3��ij��������ľ����ṹ��ͼ��ʾ����������Ļ�ѧʽΪ________��
��4���ڻ�����K3[Mn(CN)6]�У���֮����ڵ���������________(����ĸ)��
a. ���Ӽ� b. ���ۼ� c. ��λ�� d. ���
��5���Ʊ�LiMn2O4��ʵ��������£���MnO2��Li2CO3��4��1�����ʵ���֮�����ϣ���ĥ3��5 h��Ȼ�����£����¼��ȣ�����24 h����ȴ�����¡�д���÷�Ӧ�Ļ�ѧ����ʽ��____________��
����Ŀ��ij����С��ֱ�����ͼ��ʾװ�ö�ԭ��غ͵��ԭ������ʵ��̽����

��ش�
I����ͼ1��ʾװ�ý��е�һ��ʵ�顣
��1���ڱ�֤�缫��Ӧ���������£��������Cu���缫����_________������ĸ��ţ���
A���� | B��ʯī | C���� | D���� |
��2��N��������Ӧ�ĵ缫��ӦʽΪ____________________��
��3��ʵ������У�SO42-_________�����������������������������������������ƶ���
��ֽ���ܹ۲쵽��������___________________��
II����ͼ2��ʾװ�ý��еڶ���ʵ�顣ʵ������У������������������Y������Һ����Ϻ�ɫ��ֹͣʵ�飬���缫���Ա�ϸ�����Һ��Ȼ���塣�������Ϸ��֣����������FeO42-������Һ�г��Ϻ�ɫ��
��4���������У�X������Һ��pH___________������������ ������С�����������������������У�Y�������ĵ缫��ӦΪFe-6e-+8OH-=FeO42-+4H2O ��_______________��
��5������X���ռ���672 mL���壬��Y���ռ���168 mL���壨��������Ϊ��״��ʱ�������������Y�缫�����缫����������________g��