��Ŀ����
16��U��W��X��Y��Z���Ƕ�����Ԫ�أ���ԭ��������������W����̬�⻯���W������������Ӧ��ˮ������Ի��������Σ�U��X���γɳ����³�Һ̬�ķ����ң��ס��Ҿ�Ϊ������10 ���ӷ��ӣ�Y Ԫ��ԭ�ӵ� K ���������M���������ͬ��ZԪ�صĵ�����̫����ת��Ϊ���ܵij��ò��ϣ���ش����⣺��1��ZԪ�������ڱ��е�λ�õ������ڢ�A�壮
��2��X��Y��Z����Ԫ�ص�ԭ�Ӱ뾶��С�����˳����O��Si��Mg����Ԫ�ط��ű�ʾ����
��3��U��X�γɵ�18���ӻ�����ĵ���ʽ��
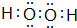 ��
����4����3���������Ļ����ﺬ�еĻ�ѧ����bc��
a�����Ӽ� b�����Թ��ۼ� c���Ǽ��Թ��ۼ�
��5����֪����N2��g��+3H2��g��=2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
��д��������ȫȼ��������̬ˮ�͵������Ȼ�ѧ����ʽ4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1266kJ/mol��
��6����������Ѱ����ʵĴ����͵缫���ϣ���W2��U2Ϊ�缫��Ӧ���HCl-NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ���д���õ�ص������缫��Ӧʽ��N2+8H++6e-=2NH4+������Һ��H+�������������������������
���� U��W��X��Y��Z���Ƕ�����Ԫ�أ���ԭ��������������W����̬�⻯���W������������Ӧ��ˮ������Ի��������Σ�ӦΪ��Σ���WΪNԪ�ء���ΪNH3��Y Ԫ��ԭ�ӵ� K ���������M���������ͬ����M�������Ϊ2����YΪMg��ZԪ�صĵ�����̫����ת��Ϊ���ܵij��ò��ϣ���ZΪSi��U��X���γɳ����³�Һ̬�ķ����ң���Ϊ������10 ���ӷ��ӣ���UΪHԪ�ء�XΪOԪ�أ���ΪH2O���ݴ˽��
��� �⣺U��W��X��Y��Z���Ƕ�����Ԫ�أ���ԭ��������������W����̬�⻯���W������������Ӧ��ˮ������Ի��������Σ�ӦΪ��Σ���WΪNԪ�ء���ΪNH3��Y Ԫ��ԭ�ӵ� K ���������M���������ͬ����M�������Ϊ2����YΪMg��ZԪ�صĵ�����̫����ת��Ϊ���ܵij��ò��ϣ���ZΪSi��U��X���γɳ����³�Һ̬�ķ����ң���Ϊ������10 ���ӷ��ӣ���UΪHԪ�ء�XΪOԪ�أ���ΪH2O��
��1��ZΪSi��Ԫ�������ڱ��е�λ�ã��������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��2��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��O��Si��Mg��
�ʴ�Ϊ��O��Si��Mg��
��3��U��X�γɵ�18���ӻ�����ΪH2O2������ʽ��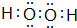 ��
��
�ʴ�Ϊ��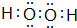 ��
��
��4��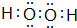 ����ԭ��֮���γɷǼ��Լ�����ԭ������ԭ��֮���γɼ��Լ���
����ԭ��֮���γɷǼ��Լ�����ԭ������ԭ��֮���γɼ��Լ���
�ʴ�Ϊ��bc��
��5����֪����N2��g��+3H2��g��=2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
�ɸ�˹���ɣ��ڡ�3-�١�2�ã�4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=3����-483.6kJ/mol��-2����-92.4kJ/mol��=-1266kJ/mol���ʷ�Ӧ�Ȼ�ѧ����ʽΪ��4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1266kJ/mol��
�ʴ�Ϊ��4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1266kJ/mol��
��6����N2��H2Ϊ�缫��Ӧ���HCl-NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ�����������ԭ��Ӧ������������ʧȥ���ӣ���������������笠����ӣ��õ�ص������缫��Ӧʽ��N2+8H++6e-=2NH4+����Һ��H+����������
�ʴ�Ϊ��N2+8H++6e-=2NH4+������
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ�����6�����������ƶ������ж�Ϊ�״��㣬ѧ��������Ϊ��������笠����ӣ��������������ƶ�����������������������笠�����������ϵ

 ��У����ϵ�д�
��У����ϵ�д� ��-C6H5 ��Br-��-COOH ��-CH3���в��ǹ����ŵ��ǣ�������
��-C6H5 ��Br-��-COOH ��-CH3���в��ǹ����ŵ��ǣ�������| A�� | �٢ۢܢޢ� | B�� | �ڢܢޢߢ� | C�� | �ڢۢݢߢ� | D�� | �٢ۢݢ� |
| A�� | CH3C��CCH3 | B�� | CH2=CH2 | C�� | CH��CCH3 | D�� | CH2=CHCH3 |
| A�� | ���ʵ���ɫ���� | B�� | ��̬�⻯��ķе㣺HCl��HBr��HI | ||
| C�� | ���ԣ�HF��HCl��HBr��HI | D�� | ���ԣ�HClO4��HBrO4��HIO4 |
| A�� | H3O+ | B�� | BF3 | C�� | CO2 | D�� | PCl5 |
| A�� | ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ��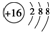 | |
| B�� | �ԭ�ӿɱ�ʾΪ${\;}_{1}^{2}$H | |
| C�� | ԭ�Ӻ�����10�����ӵ���ԭ�ӣ�${\;}_{8}^{18}$O | |
| D�� | ��λ�����ڱ���4���ڵڢ�B�� |
| A�� | C | B�� | S | C�� | O | D�� | Si |
 +O2$��_{��}^{Cu}$
+O2$��_{��}^{Cu}$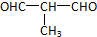 +2H2O��
+2H2O��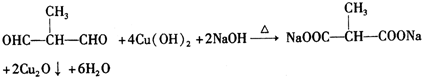 ��
��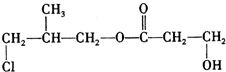
 ��
�� �ƵĻ�����������������Ӧ�ù㷺��
�ƵĻ�����������������Ӧ�ù㷺��