��Ŀ����
����Ŀ��ʵ�������÷�ͭ�Ͻ�(������������)����ȡ����ͭ����(CuSO4��5H2O)���������£�

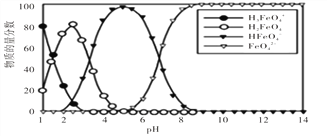
�������������������������pH�ɲο��������ݣ�
Fe3�� | Fe2�� | Cu2�� | Al3�� | |
��ʼ����ʱ��pH | 2.2 | 7.5 | 5.2 | 3.7 |
��ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 | 4.7 |
��ش�
��1�����ܽ�Ͻ�ʱ����Ļ�����2 L 3 mol��L��1�����1 L 2 mol��L��1�����϶��ɣ���Ӧ�����ɱ�״���µ�NO��������Ϊ____________L��
��2������H2O2��Ŀ����____________��
��3��Ϊ��֤��Ʒ�Ĵ��ȣ�M�������ѡ��________(����ĸ)������pH�ķ�ΧΪ____________��
a��Cu(OH)2��b��H2SO4��c��NH3��H2O��d��Na2CO3
��4����ҺD�м��������Ŀ��Ϊ_______________________________________��
��5������ҺE�Ƶ�CuSO4��5H2O��������Ҫ�IJ���Ϊ________���ᾧ�����˺��
��6��0.80g CuSO4��5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ����ȷ��200��ʱ�������ʵĻ�ѧʽ______________��

���𰸡� 44.8L ��Fe2+������Fe3+ a 4.7��PH��5.2 ����Cu2+��ˮ�� �������� CuSO4��H2O
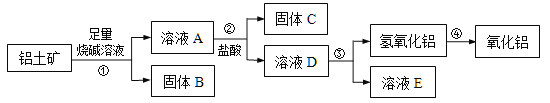
���������������̣���ʼ����������������ҺA�к���Cu2+��Fe2+��Al3+����ҺA�м�H2O2����Fe2+����ΪFe3+����ֹ����ʱӰ��Cu2+�����������Һ������M����pH��ȥFe3+��Al3+����ҺD����һϵ�в����õ�����ͭ������
��1�������⣬n(H+)=2L��3mol��L-1��2+1L��2mol��L-1=14 mol��n��NO3-��=1L��2mol��L-1=2 mol�����ݷ���ʽ3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O�ɵã������ӹ�����NO3-��ȫ��Ӧ����������ɱ�״����NO�����Ϊ2 mol��22.4Lmol-1=44.8L��
��2��������������������H2O2��Ŀ���ǽ�Fe2+����ΪFe3+��
��3�����������Һ������M����pH��ȥFe3+��Al3+��Ϊ�˲����������ʣ�M���ѡ��a.Cu(OH)2�����ݽ������������������������pH���ݿɵ���ҪʹFe3+��Al3+��ȫ��������Cu2+������������pH�ķ�ΧΪ��4.7��pH<5.2��
��4����ΪCu2+���������ӣ�����ֱ�Ӽ�����ҺD��ٽ���ˮ�⣬�ʼ�����������Cu2+ˮ�⡣
��5��������ͭ��Һ�Ƶ�CuSO45H2O���壬��Ϊ���庬�нᾧˮ��������Ҫ����������������Ũ��������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��衣
��6��CuSO45H2O���ȵ�102��ʱ��ʼ��ʧˮ��113��ʱ�ɵõ����ȶ����м�����258��ʱ�����ֽ⣬����200��ʱʧȥ��ˮ������Ϊ0.80g-0.57g=0.23g�����ݷ�Ӧ�Ļ�ѧ����ʽ��CuSO45H2O![]() CuSO4��5-x��H2O+xH2O���ɵã�
CuSO4��5-x��H2O+xH2O���ɵã�
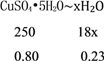
�б���ʽ�ɽ��x��4����200��ʱ�������ʵĻ�ѧʽΪCuSO4H2O��