��Ŀ����
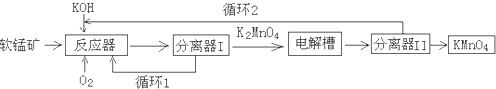
1����1��������ʡ����ѧԺ��MIT����������Ƴ�þ��Һ̬������أ��乤��ԭ����ͼ��ʾ����������ʧȥ���ӣ���ͨ�����·�������������������ӻ���ͨ������Ǩ�Ƶ������������������Ͻ������ִ���෴�Ĺ��̣�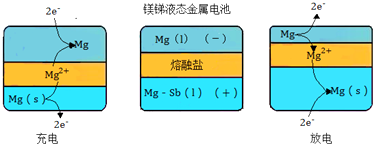
д����طŵ�ʱ��������ӦʽΪMg2++2e-=Mg��
��2���ҹ�����̲���ռ�����һλ�������Sb2S3��������Ҫ���ijұ�������������Ȼ���ˮ��Һ�ĵ���о���Ȼ�����õ��������������ɵ����Ȼ�����Ϊ���������Ի����������Խ������Ӷ�ʵ�ֽ���-���ı�·ѭ��������˴�ͳ��������С���ҵ���ϡ��ŷ���������⣮������ͼ��

��д����Ԫ�������ڱ��е�λ�õ������ڵ�VA�壮
�ڡ���ҵ���ϡ�ָ���Ƿ�������ˮ����Һ����������
�۵�������������Ҫ��Sb3+��������Sb5+����д�������ĵ缫��ӦʽSb3++3e-=Sb��
�ܸ�������ͼд�������������跢����Ӧ�Ļ�ѧ����ʽSb2S3+3SbCl5=5SbCl3+3S��
����֪����Һ�г�Sb3+�⣬����Cu2+��Pb2+���ؽ������ӣ�����c��Cu2+��=1.6��10-3mol•L-1�������Һ�м�����������Һ�е�Cu2+�պ���ȫ��������c��S2-��=8��10-40������֪Ksp��CuS��=8��10-45 Ksp��PbS��=3.4��10-28��
��ԭ�����ԭ���ǣ��ڴ���4mol•L-1��HCl��Һ�У��Դ������ƣ�Na3PO2������ԭ�����������¶ȣ�ʹAsCl3����ԭΪ��ɫ���������������ƽ�÷�Ӧ�Ļ�ѧ����ʽ��2AsCl3+3Na3PO2+3HCl+3H2O=2As��+3H3PO3+9NaCl��
���� ��1���������ӻ���ͨ������Ǩ�Ƶ������������������Ͻ�˵������ʧ�������ɵĽ��������������õ������ɽ������ʣ�
��2������Ԫ�����ڵ������ڵڢ�A�壻
�ڹ�ҵ���ϰ�����������ˮ����Һ����������
�۴�����ͼ�п��Կ�����������ΪSbCl5���������ɺ���������࣬ӦΪSb3+�����������õ������ɣ�
�ܸ�������ͼ������������������������������SbCl5��Ӧ���������SbCl5���������ԣ�Sb2S3���л�ԭ�ԣ�����������ԭ��Ӧ���ڽ���Һ�к������ʣ�������������������
��Cu2+�պ���ȫ����ʱc��Cu2+��=1��10-5������Ksp��CuS��=c��Cu2+����c��S2-��=8��10-45�����c��S2-����
�÷�Ӧ��AsCl3Ϊ������������ԭΪAs���ʣ�Na3PO2Ϊ��ԭ����������ΪH3PO3��HCl�����ԣ��ṩ���Ի��������ݵ�ʧ�����غ���ƽ��
��� �⣺��1������ʧ�������ɵĽ��������������õ������ɽ������ʣ���������Ϊþ���������缫��ӦʽΪMg2++2e-=Mg��
�ʴ�Ϊ��Mg2++2e-=Mg��
��2������Ԫ�����ڵ���Ԫ�أ�λ�����ڱ��е������ڵڢ�A�壻
�ʴ�Ϊ���������ڵ�VA�壻
�ڹ�ҵ���ϰ�����������ˮ����Һ����������
�ʴ�Ϊ����������ˮ����Һ����������
�۴�����ͼ�п��Կ�����������ΪSbCl5���������ɺ���������࣬��������ӦʽΪSb3++3e-=Sb��
�ʴ�Ϊ��Sb3++3e-=Sb��
���ڽ��������о��������Ե�SbCl5�;��л�ԭ�Ե�Sb2S3����������ԭ��Ӧ���ɲ����Ե�S���ʺ�SbCl3���ʷ�Ӧ����ʽΪSb2S3+3SbCl5=5SbCl3+3S��
�ʴ�Ϊ��Sb2S3+3SbCl5=5SbCl3+3S��
��Cu2+�պ���ȫ����ʱc��Cu2+��=1��10-5����Ksp��CuS��=c��Cu2+����c��S2-����֪c��S2-��=$\frac{Ksp��CuS��}{c��{S}^{2-}��}$=$\frac{8��1{0}^{-45}}{1��1{0}^{-5}}$=8��10-40��
�ʴ�Ϊ��8��10-40��
�������ƣ�Na3PO2������ԭ����������ΪH3PO3��PԪ����+1�۱�Ϊ+3�ۣ���̬����2��AsCl3����ԭΪAs���ʣ�AsԪ����+3�۱�Ϊ0�ۣ���̬����3������AsCl3��Na3PO2ϵ��Ϊ2��3������Ԫ���غ���ƽ��ѧ��Ӧ����ʽΪ2AsCl3+3Na3PO2+3HCl+3H2O=2As��+3H3PO3+9NaCl��
�ʴ�Ϊ��2��3��3��3��2��3��9��
���� ���⿼����ԭ��غ͵��صĹ���ԭ����Ӧ�ã����ʷ�����ᴿ�ķ����ͻ�������Ӧ�õ����ݣ�������ͼ�ǽ���Ĺؼ���

| A�� | ����ת��Ϊ��ѧ�� | B�� | �������Һ��ϡ���� | ||
| C�� | ����ͨ�����·���������� | D�� | Zn������������ |
| A�� | �衢�������衢�������ǹ�ҵ��������ά����Ҫԭ�� | |
| B�� | ���Ͳ����ͣ���֬������ | |
| C�� | ú��Һ����ʯ�ͷ����������仯 | |
| D�� | ���������ϡ�������ֽ��Ʒ���������ǿɻ��������õ���Դ |
| A�� | C+H2O=CO+H2����H=+131.2KJ/mol | |
| B�� | CO��g��+H2��g��=C��s��+H2O��g������H=-131.2KJ/mol | |
| C�� | C��s��+H2O��g��=CO��g��+H2��g������H=+10.93KJ/mol | |
| D�� | C��s��+H2O��g��=CO��g��+H2��g������H=-131.2KJ/mol |
| A�� | 1��1 | B�� | 1��2 | C�� | 2��1 | D�� | 3��1 |
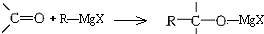 �����ò��ᆳˮ����Եõ���������ijЩ���Ӵ��ĺϳɷ���֮һ�������ϳɣ�CH3��3C-OH��������ѡ�õ�±�������ʻ�������������ȷ���ǣ�������
�����ò��ᆳˮ����Եõ���������ijЩ���Ӵ��ĺϳɷ���֮һ�������ϳɣ�CH3��3C-OH��������ѡ�õ�±�������ʻ�������������ȷ���ǣ�������| A�� | ��ȩ�������� | B�� | ��ȩ��1-����� | C�� | ��ȩ��2-����� | D�� | ��ͪ��һ�ȼ��� |
| A�� | 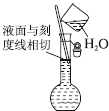 ������Һ | B�� | 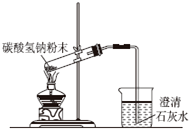 ̼���������ȷֽ� | ||
| C�� | 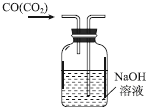 ��ȥCO�е�CO2 | D�� |  �Ʊ��ռ�����İ��� |
| A�� | X��Y���γ����ֻ��������ѧ�����Ͳ���ͬ | |
| B�� | Y�����γɻ�����YH�������ڴ������Ӽ� | |
| C�� | X������γɻ�����H2X2�������ڲ����ڼ��Լ��ͷǼ��Լ� | |
| D�� | Y2X2�����������Ӹ�����1��2 |