��Ŀ����
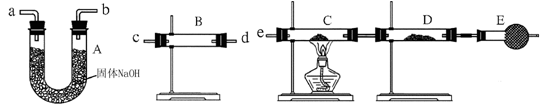
ij��ѧ̽��ѧϰС���������ͼװ����ȡ���ᣨ�гֺͼ�������������ȥ����ʵ���пɹ�ʹ�õ�ҩƷ�У�Na2CO3��NaHCO3��NH4HCO3��Na2O2��NaOH��Һ��ˮ��
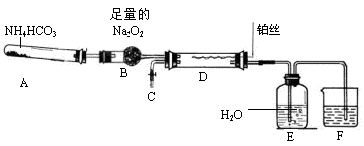
��ش��������⣺
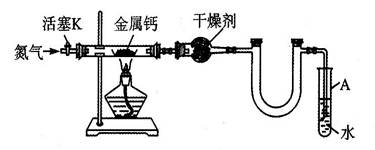
��1��װ��A�з����Ļ�ѧ��Ӧ����ʽ�� ��
��2����ȥװ��D�еļ���װ�ú�˿��Ȼ���ֺ��ȣ�������ΪD�з����Ļ�ѧ��Ӧ��һ�� ������ȡ������ȡ�����Ӧ��
��3��װ��F��ʢ�ŵ��� ��Һ���������� ��
��4��ʵ������У�ҪʹNH4HCO3���ת��ΪHNO3����Ҫ��װ��D��ͨ���������������ͬѧ������C������һ����ȡ������װ�ã���ͬѧ��Ϊ��ֱ����A���ټ��������ṩҩƷ�е�һ�����ʣ�����ҩƷ�Ļ�ѧʽ��

��ش��������⣺
��1��װ��A�з����Ļ�ѧ��Ӧ����ʽ�� ��
��2����ȥװ��D�еļ���װ�ú�˿��Ȼ���ֺ��ȣ�������ΪD�з����Ļ�ѧ��Ӧ��һ�� ������ȡ������ȡ�����Ӧ��
��3��װ��F��ʢ�ŵ��� ��Һ���������� ��
��4��ʵ������У�ҪʹNH4HCO3���ת��ΪHNO3����Ҫ��װ��D��ͨ���������������ͬѧ������C������һ����ȡ������װ�ã���ͬѧ��Ϊ��ֱ����A���ټ��������ṩҩƷ�е�һ�����ʣ�����ҩƷ�Ļ�ѧʽ��
��1��NH4HCO3 NH3��H2O��CO2��
NH3��H2O��CO2��
��2������
��3��NaOH ���տ���δ��Ӧ�ĵ��������ֹ��Ⱦ����
��4��NaHCO3
 NH3��H2O��CO2��
NH3��H2O��CO2����2������
��3��NaOH ���տ���δ��Ӧ�ĵ��������ֹ��Ⱦ����
��4��NaHCO3
��ȡ�����漰��ӦΪ��
4NH3��5O2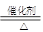 4NO��6H2O 2NO��O2=2NO2 3NO2��H2O=2HNO3��NO
4NO��6H2O 2NO��O2=2NO2 3NO2��H2O=2HNO3��NO
����װ�ü�ҩƷ�ɿ�����NH4HCO3 NH3��H2O��CO2�� 2Na2O2��2H2O=4NaOH��O2�� 2Na2O2��2CO2=2Na2CO3��O2
NH3��H2O��CO2�� 2Na2O2��2H2O=4NaOH��O2�� 2Na2O2��2CO2=2Na2CO3��O2
��1��NH4HCO3 NH3��H2O��CO2��
NH3��H2O��CO2��
��2�������Ĵ�������4NH3��5O2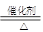 4NO��6H2OΪ���ȷ�Ӧ
4NO��6H2OΪ���ȷ�Ӧ
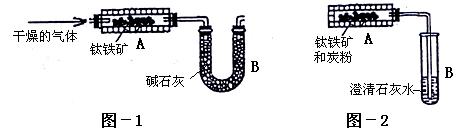
��3����Ӧ���������ɿ���Ⱦ������NO��NO2��һ����ü�Һ��������
��4�����Լ���NaHCO3��ͨ����ֽ����ɵ�H2O��CO2������Na2O2��Ӧ�õ�����
4NH3��5O2
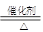 4NO��6H2O 2NO��O2=2NO2 3NO2��H2O=2HNO3��NO
4NO��6H2O 2NO��O2=2NO2 3NO2��H2O=2HNO3��NO����װ�ü�ҩƷ�ɿ�����NH4HCO3
 NH3��H2O��CO2�� 2Na2O2��2H2O=4NaOH��O2�� 2Na2O2��2CO2=2Na2CO3��O2
NH3��H2O��CO2�� 2Na2O2��2H2O=4NaOH��O2�� 2Na2O2��2CO2=2Na2CO3��O2��1��NH4HCO3
 NH3��H2O��CO2��
NH3��H2O��CO2����2�������Ĵ�������4NH3��5O2
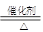 4NO��6H2OΪ���ȷ�Ӧ
4NO��6H2OΪ���ȷ�Ӧ��3����Ӧ���������ɿ���Ⱦ������NO��NO2��һ����ü�Һ��������
��4�����Լ���NaHCO3��ͨ����ֽ����ɵ�H2O��CO2������Na2O2��Ӧ�õ�����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
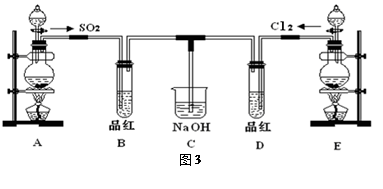
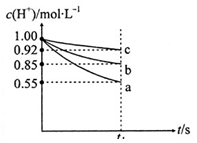
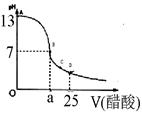
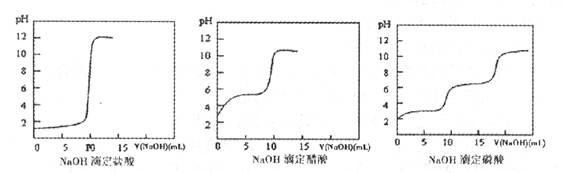
 C6H12O6�������ǣ�+6O2��
C6H12O6�������ǣ�+6O2��