��Ŀ����
��9�֣�
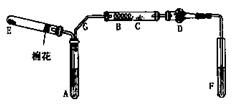
ij��ѧ��һ��ѧ��ȤС���ͬѧ����ʵ����̽���ù�����п��Ũ���ᷴӦ��ȡSO2��
(1)������ȡ��SO2�п��ܺ��е������� �� ��
(2)ijͬѧ������װ�����ӳ�һ����ʵ��װ������֤�ٵ��ж��Ƿ���ȷ�������������������ʱ�����������ĸ�װ�õ��ܵı�������� �� �� �� �� �� (��a��b������д��ÿ��װ��ֻ����ʹ��һ��)��
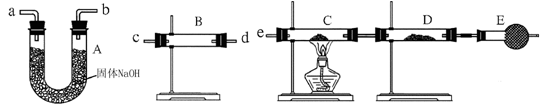
(3)���ݢ�ȷ����ʵ����̣��뽫ʵ��ʱ�й�װ������ʢҩƷ��ʵ�������������±���
ij��ѧ��һ��ѧ��ȤС���ͬѧ����ʵ����̽���ù�����п��Ũ���ᷴӦ��ȡSO2��
(1)������ȡ��SO2�п��ܺ��е������� �� ��
(2)ijͬѧ������װ�����ӳ�һ����ʵ��װ������֤�ٵ��ж��Ƿ���ȷ�������������������ʱ�����������ĸ�װ�õ��ܵı�������� �� �� �� �� �� (��a��b������д��ÿ��װ��ֻ����ʹ��һ��)��

(3)���ݢ�ȷ����ʵ����̣��뽫ʵ��ʱ�й�װ������ʢҩƷ��ʵ�������������±���
| װ�� | ��ʢҩƷ | ʵ������ | ���� |
| B | | | |
| C | CuO���� | | |
| D | ��ˮCuSO4 | |
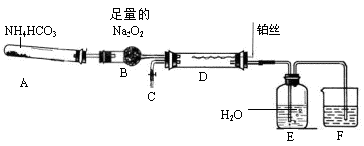
(1)H2 H2O(g) (2) c��d��a��b��e����c��d��a��b�ӿڿɶԵ�����
(3)
(3)
| װ�� | ��ʢҩƷ | ʵ������ | ���� |
| B | ��ˮCuSO4 | ��ɫ��ĩ�����ɫ | �����к���ˮ���� |
| C | | ��ɫ�����ɺ�ɫ | �����к������� |
| D | | ��ɫ��ĩ�����ɫ |
��Ҫ��������ļ���
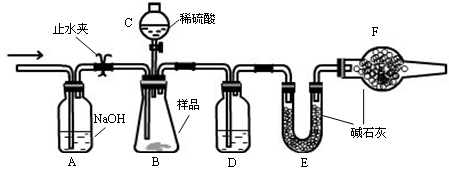
��1���漰��Ӧ��Zn��2H2SO4(Ũ)=ZnSO4��SO2����2H2O Zn��H2SO4(ϡ)=ZnSO4��H2��������������г�SO2�⣬�����ܺ���������ˮ����
��2��һ��������������ˮ����ͭ������ˮ�����Ĵ��ڣ�ѡ��Bװ�ã��������ɲ��û�ԭCuO������ˮ���ٴ�����ˮ����ͭ���������ɵ�ˮ������Bװ�ú�����A����NaOH������ˮ���������CDEװ�ã���������ԭװ��C�е�CuO�����ɵ�ˮ��D�е���ˮ����ͭ�����飬��Ϊ�˷�ֹ�����е�ˮ������Ӱ�죬E�п�װ�м�ʯ�ҵȸ����
�ʽӿ�˳��Ϊc��d��a��b��e
(3)
��1���漰��Ӧ��Zn��2H2SO4(Ũ)=ZnSO4��SO2����2H2O Zn��H2SO4(ϡ)=ZnSO4��H2��������������г�SO2�⣬�����ܺ���������ˮ����
��2��һ��������������ˮ����ͭ������ˮ�����Ĵ��ڣ�ѡ��Bװ�ã��������ɲ��û�ԭCuO������ˮ���ٴ�����ˮ����ͭ���������ɵ�ˮ������Bװ�ú�����A����NaOH������ˮ���������CDEװ�ã���������ԭװ��C�е�CuO�����ɵ�ˮ��D�е���ˮ����ͭ�����飬��Ϊ�˷�ֹ�����е�ˮ������Ӱ�죬E�п�װ�м�ʯ�ҵȸ����
�ʽӿ�˳��Ϊc��d��a��b��e
(3)
| װ�� | ��ʢҩƷ | ʵ������ | ���� |
| B | ��ˮCuSO4 | ��ɫ��ĩ�����ɫ | �����к���ˮ���� |
| C | | ��ɫ�����ɺ�ɫ | �����к������� |
| D | | ��ɫ��ĩ�����ɫ |

��ϰ��ϵ�д�
�����Ŀ
 �ⶨ������������
�ⶨ������������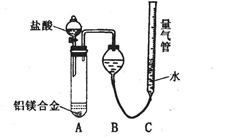

 = 2��70��10-39]
= 2��70��10-39]
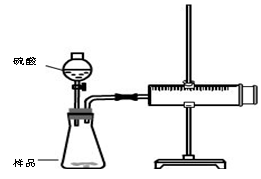
 ������Ա���ǿ�����ܷ�Ӧ���ɰ�ɫBaSO3����������ͼ��ʾװ�ý���ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩
������Ա���ǿ�����ܷ�Ӧ���ɰ�ɫBaSO3����������ͼ��ʾװ�ý���ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩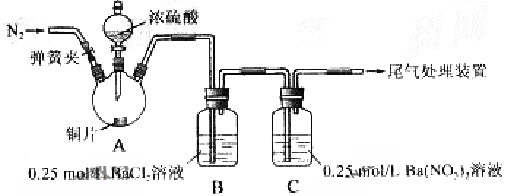
 ������ɫ������Һ���Ϸ�����dz��ɫ������ʧ
������ɫ������Һ���Ϸ�����dz��ɫ������ʧ