��Ŀ����
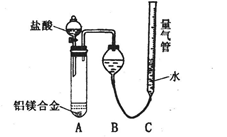
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��
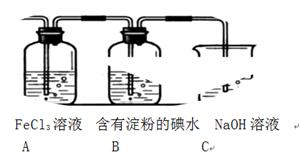
��1��SO2���廹ԭFe3+�IJ����� �������ӷ��ţ����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮���� ��
��2������ʵ�鷽����������ʵ������ȡ����SO2���� ������ţ���
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D���������ڸ�������O2��Ӧ
��3��װ��C�������� ��
��4����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ��������� ������ţ���
A�������� B��ʯ���� C��©�� D���ձ� E�������� F������
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ��ԭ���� ��
��6���ܱ���I���Ļ�ԭ������SO2�������� ��

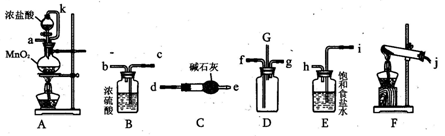
��1��SO2���廹ԭFe3+�IJ����� �������ӷ��ţ����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮���� ��
��2������ʵ�鷽����������ʵ������ȡ����SO2���� ������ţ���
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D���������ڸ�������O2��Ӧ
��3��װ��C�������� ��
��4����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ��������� ������ţ���
A�������� B��ʯ���� C��©�� D���ձ� E�������� F������
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ��ԭ���� ��
��6���ܱ���I���Ļ�ԭ������SO2�������� ��
��16�֣���1��SO42-��Fe3+�� 1�U2 ��2��B
��3������SO2β������ֹ��Ⱦ���� ��4��BF
��5�������٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ
��6��B����ɫ��Һ��ɫ ��ÿ��2�֣�
��3������SO2β������ֹ��Ⱦ���� ��4��BF
��5�������٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ
��6��B����ɫ��Һ��ɫ ��ÿ��2�֣�
�����������1��SO2���廹ԭFe3+�IJ�����SO42-��Fe3+����Ӧ�ķ���ʽ��SO2��2Fe3����2H2O=2Fe2����SO42����4H�����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮��1�U2��
��2��������������ԣ����������Ʒ�Ӧ�ò���SO2��Na2SO3������Ũ���ᷴӦ����SO2��B��ȷ��ѡ��CD����Ȼ��������SO2�����õ������岻�Ǵ�������ѷ��룬��ѡB��
��3��SO2�Ǵ�����Ⱦ�����Cװ�õ�����������SO2β������ֹ��Ⱦ������
��4����Һ����Ũ��Ӧ���������������������Ҳ���Ҫ��ʯ��������˴�ѡBF��
��5������A����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ�����Է������Dz������ġ�
��6��SO2��ʹ���ʵԭ����B����ɫ��Һ��ɫ�Ϳ���˵��I���Ļ�ԭ������SO2�ġ�2����̽��ʵ����й��ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��ʵ�������ĺͽ��ⷽ����ָ����ѵ��������������ѧ���淶���Ͻ���ʵ����ƺ���������������ѧ����ѧ�����������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
�����Ŀ
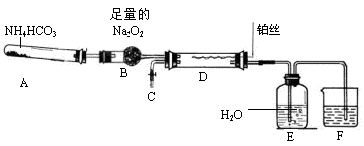
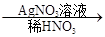 ��ɫ��������ش��������⣺
��ɫ��������ش��������⣺ ��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
 �ⶨ������������
�ⶨ������������