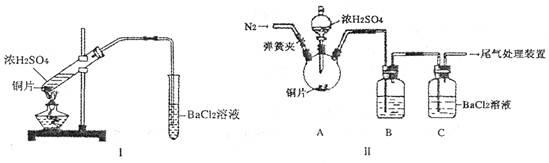
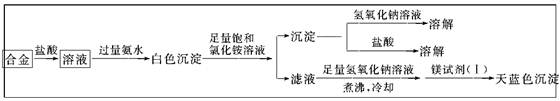
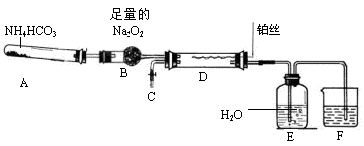
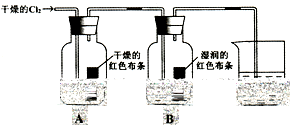
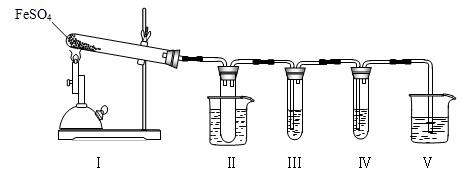
��Ŀ����
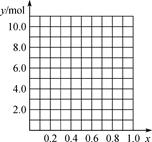
��12�֣�ijѧ����ʵ���Ҳⶨһδ֪Ũ�ȵ�ϡ����,��֪��25ml�������Ʊ���Һ����μ���0.2mol/L������Һ��PH�仯������ͼ��ʾ��
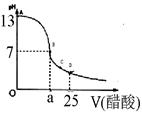
��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����="��" )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
�������Ũ��ԼΪ___________________ (������λ��Ч����)��
�ζ��ﵽ�յ�ı�־��

��1��������������Һ�����ʵ���Ũ��Ϊ mol.L��1
��2����B�㣬a 12.5ml(�>������<����="��" )��
��3������100 mL NaOH����Һ����������������ƽ������������ͷ�ι��⣬����Ҫ
��4���� ��ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20.00 mL������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�ζ��ﵽ�յ�ı�־��
����12�֣�
��1��0.1mol/L ��2���� ��3���ձ���100mL����ƿ�����һ����1�֣�
��4����ʽ�ζ��� 0.12 mol��L��1 ���һ������������Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ
��1��0.1mol/L ��2���� ��3���ձ���100mL����ƿ�����һ����1�֣�
��4����ʽ�ζ��� 0.12 mol��L��1 ���һ������������Һ���룬��Һ����ɫǡ�ñ��dz��ɫ��������ڲ���ɫ
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

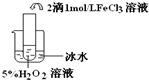
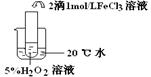
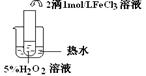
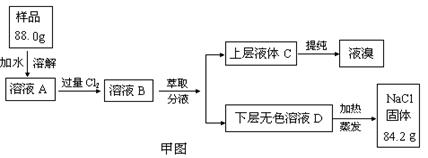
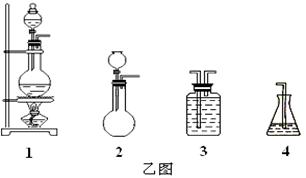
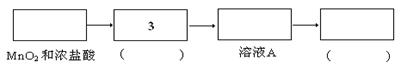
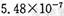 ��������������
��������������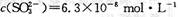 ��
��