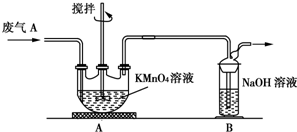
��Ŀ����
1������������ȫ��ȷ��һ���ǣ�������������Ħ�����ԼΪ22.4L•mol-1
�ڱ�״���£�22.4Lˮ������������ΪNA
��100mL O.1mol•L-1��H2S03��Һ�У����е�������ԼΪO.03NA
�ܳ��³�ѹ�£�1.6gO3������ԭ����ΪNA
��9g D2O�к��еĵ�����Ϊ5NA
������Ħ������Ϊ8g��
| A�� | �٢ۢܢ� | B�� | �ܢݢ� | C�� | �� | D�� | �٢� |
���� ������Ħ�������������״̬�йأ�
�ڱ�״���£�ˮΪҺ̬��
��S032-��ˮ�⣬��ʹ��Һ�е����Ӹ������ࣻ
��O3����ԭ�ӹ��ɣ�
��D2O��Ħ������Ϊ20g/mol��
��Ħ������Ϊg/mol��
��� �⣺������Ħ�������������״̬�йأ���һ����22.4L/mol���ʴ���
�ڱ�״���£�ˮΪҺ̬���ʲ��ܸ�������Ħ����������������ʵ������ʴ���
��S032-��ˮ�⣬��ʹ��Һ�е����Ӹ������࣬�ʺ��е�����������O.03NA���ʴ���
��O3����ԭ�ӹ��ɣ���1.6g�����е���ԭ�ӵ����ʵ���n=$\frac{1.6g}{16g/mol}$=0.1mol������ΪNA��������ȷ��
��D2O��Ħ������Ϊ20g/mol����9g��ˮ�����ʵ���n=$\frac{9g}{20g/mol}$=0.45mol����1mol��ˮ�к�10mol���ӣ���0.45mol��ˮ�к�4.5mol���Ӽ�4.5NA�����ʴ���
��Ħ������Ϊg/mol������Ϊ��ԭ�ӷ��ӣ��ʺ�������Է�������Ϊ4���ʺ�����Ħ������Ϊ4g/mol���ʴ���
��ѡC��
���� ���⿼���˰���٤���������йؼ��㣬�������չ�ʽ��ʹ�ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
15��һ�������·�Ӧ 2AB��g���TA2��g��+B2��g���ﵽƽ��״̬�ı�־�ǣ�������
| A�� | �����л���������������ʱ��仯 | |
| B�� | �������������� AB��A2��B2���� | |
| C�� | v����AB��=2v����A2�� | |
| D�� | ��λʱ��������n mol A2��ͬʱ����2n mol AB |
9���ܱ������н������·�Ӧ��X2��g��+3Y2��g��?2Z��g����X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.2mol/L��0.6mol/L��0.4mol/L���ﵽƽ��ʱ���������ݿ϶���ȷ���ǣ�������
| A�� | X2Ϊ0.4 mol/L��Y2Ϊ1.2 mol/L | B�� | Y2Ϊ1.6 mol/L | ||
| C�� | X2Ϊ0.3 mol/L��ZΪ0.2 mol/L | D�� | ZΪ1.0 mol/L |
6��ͨ�����������Ļ�ѧҩƷ������ǡ���ķ����ᴿ��������ȥij��Һ���ܽ�����ʣ����������в���ȷ���ǣ������ڵ�����Ϊ���ʣ���������
| A | KCl��Һ��CaCl2�� | ��K2CO3��Һ�����ˣ��ټ��������� |
| B | NaNO3��Һ��AgNO3�� | ������NaCl��Һ������ |
| C | NaCl��Һ��I2�� | �Ӿƾ�����Һ |
| D | KBr��Һ��Br2�� | ��CCl4����Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
13�����ʽ��ͬ�����Ȳ���ͬϵ��ֲ���ͬ���칹����ǣ�������
����ϩ��3-��-1-��ϩ�� �ڱ�����Ȳ �۱����Ѻͱ��״��� �ܼ�ȩ�ͼ��������
����ϩ��3-��-1-��ϩ�� �ڱ�����Ȳ �۱����Ѻͱ��״��� �ܼ�ȩ�ͼ��������
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �ڢ� |
10��������24g��3mol•L-1NaOH��Һ���������Ƴ�1mol•L-1NaOH��Һ��Ӧȡԭ��Һ������ˮ�������ԼΪ��������
| A�� | 1��2 | B�� | 1��3 | C�� | 2��1 | D�� | 2��3 |
11�����з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A�� | С�������Ͷ��ˮ�У�Na+2H2O=Na++2OH-+H2�� | |
| B�� | Fe��������Һ�ķ�Ӧ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ʯ��ʯ�������CaCO3+2H+�T�TCa2++H2O+CO2�� | |
| D�� | пƬ������������Һ�У�Zn+Ag+�TZn2++Ag |
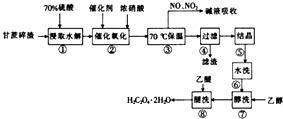 ������һ����Ҫ�Ļ���ԭ�ϣ��������л��ϳɣ������ڶ�Ԫ���ᣬ�������Ҵ���ˮ���������ѣ��Ը�����������Ҫ�ɷ�Ϊ��ά�أ�Ϊԭ����ȡ����Ĺ����������£�
������һ����Ҫ�Ļ���ԭ�ϣ��������л��ϳɣ������ڶ�Ԫ���ᣬ�������Ҵ���ˮ���������ѣ��Ը�����������Ҫ�ɷ�Ϊ��ά�أ�Ϊԭ����ȡ����Ĺ����������£�