��Ŀ����
16�����Ṥҵ�����ķ���A����Ҫ�ɷ֣�SO2��O2��N2��CO2�ȣ��ŷŵ������л���Ⱦ������ij��ѧ��ȤС��Է���A����ɽ���̽������ش��������⣮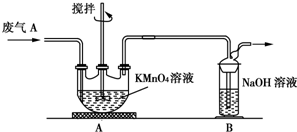
��1��ͬѧ�����ʵ��������A�к���CO2��Ӧѡ����Լ���BC��
A��NaOH��Һ B������KMnO4��Һ C������ʯ��ˮ D������
��2��ͬѧ�����ⶨ����A��SO2�ĺ�����ȡa L����A���������TUװ�ý���ʵ�飮
��Ϊ�˱�֤ʵ��ɹ���װ��AӦ���е�������KMnO4��Һ���Ϻ�ɫ������ȫ��ɫ�������Ŀ�������SO2�������ʣ�
��ͬѧ����ΪAװ��ǰ������������a L�����к��е�SO2��������Ȼ����к������㣮��С�����ۺ�ͬѧ��������ʣ�����ͬѧ����ʵ�飬��������SO2��ȫ�����գ������ⶨ�Ľ��Ӧ��ƫ��ͬѧ���ƶϵ�������2H2SO3+O2=2H2SO4��
���� ��1�����ʵ��������A�к��е�CO2����Ҫ��ȥ������������Ŷ�����̼�ļ��飬�����������������ͨ������ʯ��ˮ���������̼�Ĵ��ڣ�
��2���ٲⶨ����A��SO2�ĺ�����Ϊ�˱�֤ʵ��ɹ���Ҫ��Aװ�����ø��������Һȫ�����գ����������Һ����ɫ֤��������ȫ���������Ҫ�����dz�����ն���������������ʣ�
�ڲⶨ�Ľ��Ӧ��ƫ���������ɵ������ᱻ����������������������������
��� �⣺��1�����Ṥҵ�����ķ���A����Ҫ�ɷ֣�SO2��O2��N2��CO2�ȣ��ŷŵ������л���Ⱦ���������ʵ��������A�к��е�CO2����Ҫ��ȥ������������Ŷ�����̼�ļ��飬�����������������ͨ������ʯ��ˮ���������̼�Ĵ��ڣ�ʯ��ˮ�����֤�����ж�����̼������A��Ϊ���������Һ��B��Ϊ����ʯ��ˮ��
�ʴ�Ϊ��BC��
��2����Ϊ�˱�֤ʵ��ɹ���Ҫ��Aװ�����ø��������Һȫ�����գ����������Һ����ɫ֤����������������ȫ���������Ҫ�����dz�����ն���������������ʣ�
�ʴ�Ϊ��KMnO4��Һ���Ϻ�ɫ������ȫ��ɫ�����SO2�������ʣ�
�ڰ���ͬѧ����ʵ�飬��������SO2��ȫ�����գ��ⶨ�Ľ��Ӧ��ƫ���������ɵ������ᱻ����������������ʵ������з���2H2SO3+O2=2H2SO4�����²ⶨ������������
�ʴ�Ϊ��2H2SO3+O2=2H2SO4��
���� ���⿼����������ɵ�ʵ��̽���������̷����жϣ���Ҫ���������ʵ�����Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| �롡�� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��Ϊ����SO2���ŷţ���ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����ab������ĸ����
a��Ca��OH��2������b��Na2CO3������c��CaCl2������d��NaHSO3
��3������֪����������NO�ķ�ӦΪN2��g��+O2��g��?2NO��g����H��0�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ�������ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���NO�ĺ�������
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO��g���T2C��s��+O2��g����
��֪�÷�Ӧ�ġ�H��0�������������ܷ�ʵ�ֵ����ݣ���H��0��S��0�������κ��¶��¡�G��0���������Է����У�
| A�� | 1 L 0.1 mol/L���� | B�� | 0.1 L 0.1 mol/L H3PO4��Һ | ||
| C�� | 0.5 L 0.1 mol/L���� | D�� | 2 L 0.1 mol/L H2SO3��Һ |
������Ħ�����ԼΪ22.4L•mol-1
�ڱ�״���£�22.4Lˮ������������ΪNA
��100mL O.1mol•L-1��H2S03��Һ�У����е�������ԼΪO.03NA
�ܳ��³�ѹ�£�1.6gO3������ԭ����ΪNA
��9g D2O�к��еĵ�����Ϊ5NA
������Ħ������Ϊ8g��
| A�� | �٢ۢܢ� | B�� | �ܢݢ� | C�� | �� | D�� | �٢� |
| A�� | ��ɫ��Һ�У�Al3+��Cl-��MnO4-��SO42- | |
| B�� | ���д���HCO3-����Һ�У�Na+��Ca2+��NO3-��Cl- | |
| C�� | 0.1mol•L-1AgNO3��Һ��H+��K+��SO42-��Cl- | |
| D�� | ʹʯ����ɫ����Һ��CH3COO-��Cl-��NO3-��K+ |

| A�� | 95��ʱ��pH=4��H2SO4��Һ�У���ˮ�������c��OH-��=1��10-10mol/L | |
| B�� | 15��ʱ��pH=7����Һ������ | |
| C�� | AB�����ϵĵ����ʾ������Һ | |
| D�� | B���Ӧ���¶ȸ���A���Ӧ���¶� |

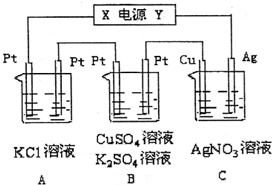 ��ͼ��ʾ�������5minʱ��ͭ�缫��������2.16g���Իش�
��ͼ��ʾ�������5minʱ��ͭ�缫��������2.16g���Իش�