��Ŀ����
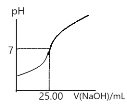
����Ŀ���Ȼ����Ǻϳ������ȣ�SO2Cl2���ij��÷�����ʵ���Һϳ������ȣ�SO2Cl2���ķ�Ӧ��ʵ��װ�����£�
SO2(g)��Cl2(g) ![]() SO2Cl2(l) ��H��- 92.7 kJ/mol
SO2Cl2(l) ��H��- 92.7 kJ/mol
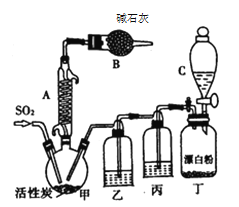
�й���Ϣ���£�������ͨ��������Ϊ��ɫҺ�壬�۵�Ϊ��54.1 �棬�е�Ϊ69.1 �棬�ܶ�Ϊ1.67g /cm3���ڳ�ʪ����������������100�����Ͽ�ʼ�ֽ⣬���ɶ�����������������ڷ���Ҳ�ᷢ���ֽ⡣�ش��������⣺
��1��װ�ü�������A������Ϊ___________�����л���̿��������________��B������Ϊ_________��
��2��װ�ö��з�����Ӧ�����ӷ���ʽΪ__________________________��
��3��װ�ñ��е��Լ�Ϊ____________________����ȱ��װ���ң������Ͷ���������ܷ�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��4��Ϊ��߱�ʵ���������ȵIJ��ʣ���ʵ���������Ҫע���������_______������ţ���
����ͨ����ˮ����ͨ�� �ڿ����������ʣ��������˿�
����������ƿ���̣����ʵ����� �ܼ���������ƿ
��5����ʵ����ͨ��SO2�����Ϊ11.2 L��������ɱ�״������Cl2���㣬ʵ�������ռ���������27.0 mL���������ȵIJ���Ϊ_________________���������һλС������
���𰸡������ܣ����������ܻ��������ܣ� ���� ��ֹˮ�������룬����β�� Cl+ClO+2H+��Cl2��+H2O ����ʳ��ˮ�����Ȼ�����Һ�� SO2+Cl2+2H2O��H2SO4+2HCl �٢ڢ� 66.8%
��������
��1�����������ص㣬װ�ü�������A������Ϊ���������ܻ��������ܣ�����̿���Ƿ�Ӧ�����������B�еļ�ʯ�ҿɷ�ֹ�����е�ˮ��������װ�ã������Է�ֹ�ж�����������������β��������
��2��װ�ö�������Ư�������ᷴӦ��ȡ������
��3��װ�ñ�Ϊ��ȥ�����е�HCl���壻װ���ҵ��Լ�ΪŨ���ᣬ�ɷ�ֹˮ��������װ�ü��У�����SO2Cl2�ڳ�ʪ����������������
��4������ͨ����ˮ����ͨ�����ɼ���SO2Cl2�Ļӷ���
�ڿ����������ʣ��������˿죬��ʹ��Ӧ��ֽ��У�
��100������SO2Cl2��ʼ�ֽ⣬���ɶ����������������������ƿ���̣����ʵ����£��ɼ���SO2Cl2�ķֽ⣻
�ܼ���������ƿ���ֽ��������ʽ��ͣ�
��5������n(SO2):n(SO2Cl2)=1��1��ת����=![]() ��100%���м��㡣
��100%���м��㡣
��1�����������ص㣬װ�ü�������A������Ϊ���������ܻ��������ܣ�����̿���Ƿ�Ӧ�����������B�еļ�ʯ�ҿɷ�ֹ�����е�ˮ��������װ�ã������Է�ֹ�ж�����������������β��������
��2��װ�ö�������Ư�������ᷴӦ��ȡ��������Ӧ�����ӷ���ʽΪCl+ClO+2H+��Cl2��+H2O��
��3��װ�ñ�Ϊ��ȥ�����е�HCl���壬ʹ�õ��Լ�Ϊ����ʳ��ˮ��װ���ҵ��Լ�ΪŨ���ᣬ�ɷ�ֹˮ��������װ�ü��У�����SO2Cl2�ڳ�ʪ������������������Ӧ�ķ���ʽΪSO2+Cl2+2H2O��H2SO4+2HCl��
��4������ͨ����ˮ����ͨ�����ɼ���SO2Cl2�Ļӷ����Ӷ���߲��ʣ��������⣬����ȷ��
�ڿ����������ʣ��������˿죬��ʹ��Ӧ��ֽ��У��Ӷ���߲��ʣ��������⣬����ȷ��
��100������SO2Cl2��ʼ�ֽ⣬���ɶ����������������������ƿ���̣����ʵ����£��ɼ���SO2Cl2�ķֽ⣬�Ӷ���߲��ʣ��������⣬����ȷ��
�ܼ���������ƿ���ֽ��������ʽ��ͣ������ⲻ�����ܴ���
��Ϊ�٢ڢۣ�
��5�����ݷ���ʽSO2(g)��Cl2(g) ![]() SO2Cl2(l)��n(SO2):n(SO2Cl2)=1��1��m(SO2Cl2)=1.67g /cm3��27.0 mL=45.09g��n(SO2Cl2)=
SO2Cl2(l)��n(SO2):n(SO2Cl2)=1��1��m(SO2Cl2)=1.67g /cm3��27.0 mL=45.09g��n(SO2Cl2)=![]() =0.334mol����Ӧ��n(SO2) =0.334mol��ת����=
=0.334mol����Ӧ��n(SO2) =0.334mol��ת����=![]() ��100%=66.8%��
��100%=66.8%��

����Ŀ��̼�塢����Ԫ�ؼ��仯����������������ͻ���������Ӧ�ù㷺��
��1�������й��������ʵݱ�������У���ȷ����________________������ţ���
A�����ԣ�HNO3>H2CO3>H2SiO3 B���ȶ��ԣ�NH3>AsH3>PH3
C���ȶ��ԣ�N2<P4�����ף� D�������£�ͬŨ����Һ��pH��Na3PO4<Na3AsO4
��2����֪��
(a)CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H1=��890.3 kJ��mol1
(b)2H2(g)+O2(g)=2H2O(l) ��H2=��571.6 kJ��mol1
(c)C(s)+O2(g)=CO2(g) ��H3=��393.5 kJ��mol1
��C(s)+2H2(g)=CH4(g) ��H=___________kJ��mol1��
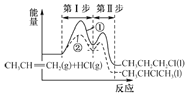
��CH4�Ʊ��ϳ�����ԭ����CH4(g)+CO2(g) ![]() 2CO(g)+2H2(g)��
2CO(g)+2H2(g)��
A. �����������ܱ�ʾ�÷�Ӧ�ں��º��������´ﵽƽ��״̬����____��
a. ��������ѹǿ���ٷ����仯
b. ���������ܶȲ��ٷ����仯
c. ��Ӧ������CO2��CO�����ʵ����ı�ֵ���ٷ����仯
d. ��������ƽ����Է����������ٷ����仯
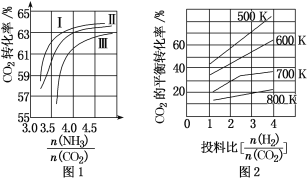
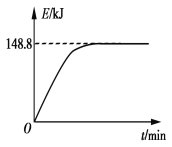
B. ��1 g CH4(g)��ȫ��Ӧ��������Ϊ15.5 kJ���ں����ܱ������г���1 mol CH4��1 mol CO2��һ�������·�Ӧ����ϵ������������ʱ��仯��ͼ��ʾ���ڸ������£���������ת���ʣ�����Ϊ____________��
��3�����ܱ������з�����Ӧ��2NO(g)+H2(g)= N2(g)+2H2O(g)���䷴Ӧ������Ũ�ȹ�ϵʽΪv=kcm(NO)��cn(H2)��kΪ������ֻ���¶��йأ�m��nΪ��Ӧ������ȡ������������ij�¶��²���й����������ʾ��
���� | c(NO)/(mol��L1) | c(H2)/(mol��L1) | v/(mol��L1��min1) |
�� | 0.10 | 0.10 | 0.414 |
�� | 0.20 | 0.20 | 3.312 |
�� | 0.10 | 0.20 | 0.828 |
�ܷ�Ӧ���������У���i����2NO+H2=N2+H2O2(����)����ii����H2O2+H2![]() 2H2O���ܿ죩���������¶��£���c(NO)=c(H2)=0.50 mol��L1ʱv=_________mol��L1��min1��
2H2O���ܿ죩���������¶��£���c(NO)=c(H2)=0.50 mol��L1ʱv=_________mol��L1��min1��
��4����2 L�����ܱ������г���3 mol NO(g)��3 mol CO(g)��������Ӧ��2NO(g)+2CO(g) ![]() N2(g)+2CO2(g)����һ���¶��´ﵽƽ�⣬���ƽ����ϵ��c(N2)=0.5 mol��L1�����������ƽ�ⳣ��KֵΪ_______����Ӧǰ������������ѹǿ֮��Ϊ_________________________��
N2(g)+2CO2(g)����һ���¶��´ﵽƽ�⣬���ƽ����ϵ��c(N2)=0.5 mol��L1�����������ƽ�ⳣ��KֵΪ_______����Ӧǰ������������ѹǿ֮��Ϊ_________________________��