��Ŀ����
17����������11�����ʣ��밴Ҫ��ش����⣺��1������ ��2��п ��3�������� ��4��̼ ��5������ ��6���������� ��7���Ȼ��� ��8������������Һ��9������ ��10�����Ȼ�̼ ��11��̼����
��������״���෨���������ʽ��з��ࣨ���û�ѧʽ��ʾ��д��ѧʽ����Ҫ���������������ͣ����ο���ͼ�����࣬��������������ͼ����ײ㣬�м��εĺ�����д���ʵ����
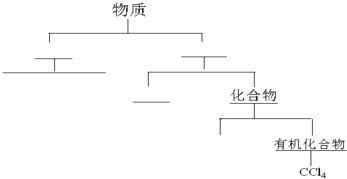
����ˮ�к����������ʣ����Ρ�þ�Ρ������Σ����ֽ����ᴿ�Ի�ô�����ʳ��ˮ��
���ڢ��������ѡ����ʵ��Լ����������̽��г��ӣ����ش��й����⣮
����ˮ$\stackrel{��NaOH}{��}$$��_{����A}^{��}$ $��_{����A}^{��}$$��_{����A}^{��}$������ʳ��ˮ
��1��������������Ļ�ѧ��Ӧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl��Na2CO3+BaCl2=BaCO3��+2NaCl
��2������A�������ǹ���
��3������ܼ�����Լ����������������ᣬ��δ���в���A�ͼӸ��Լ��ٲ����Ľ�������ɵ�̼���γ����������ᣬ����ȥCa2+��Ba2+_��
��4������������Լ�Ӧ���ǹ����ģ��жϸ��Լ������ķ����ǣ�������Һ�����ֲ�����ϲ���Һ�����μ�Na2CO3��Һ�������ٲ�����������˵�����Լ��Ѿ�������
���� I�����ʷ�Ϊ������ͻ����������Ϊ�����ʺͻ�����������Ϊ�л������������������������Ϊ������Լ�������ݴ˽�ɣ�
II����1�������ڴ��ε��ᴿ�����У�BaCl2�ļ��������Na2CO3֮ǰ���ʼ���Ģ�ΪBaCl2����ΪNa2CO3������Na2CO3��Ŀ���dz�ȥBaCl2��CaCl2���ݴ�д����ѧ����ʽ��
��2���ڼ���NaOH��BaCl2��Na2CO3֮��Ҫ�ȹ��ˣ��ټ������������ᣬ�ʢ������ᣬ����A�ǹ��ˣ�
��3������ܼ�����Լ������ᣬ��������˾ͼ����ᣬ���ʹ��ǰ���ɵ�Mg��OH��2��BaCO3��CaCO3�ܽ⣻
��4��Na2CO3����������Һ���й�����CO32-�������μ�Na2CO3��Һ�������ٲ�����������˵�����Լ��Ѿ�������
��� �⣺��1���������ڻ�����еĽ��壬��2��пΪ�������ʣ���3��������Ϊ�������е������� ��4��̼Ϊ�ǽ������ʣ���5���������Ȼ����ˮ��Һ�����ڻ�����е���Һ����6�������������ڼ�ǻ������7���Ȼ����ǻ������е��Σ���8������������ҺΪ������������Һ����9������Ϊ�ᣬ��10�����Ȼ�̼Ϊ�л����11��̼����Ϊ�Σ��ʴ�Ϊ��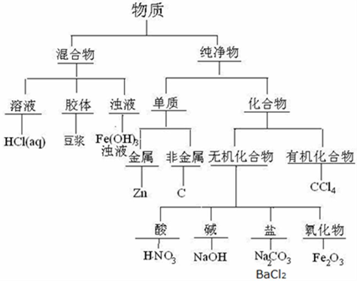
��1�������ڴ��ε��ᴿ�����У�BaCl2�ļ��������Na2CO3֮ǰ���ʼ���Ģ�ΪBaCl2����ΪNa2CO3������Na2CO3��Ŀ���dz�ȥBaCl2��CaCl2���ʻ�ѧ����ʽΪ��
BaCl2+Na2CO3=BaCO3��+2NaCl��CaCl2+Na2CO3=CaCO3��+2NaCl���ʴ�Ϊ��BaCl2+Na2CO3=BaCO3��+2NaCl��CaCl2+Na2CO3=CaCO3��+2NaCl��
��2���ڼ���NaOH��BaCl2��Na2CO3֮��Ҫ�ȹ��ˣ��ټ������������ᣬ�ʢ������ᣬ����A�ǹ��ˣ��ʴ�Ϊ�����ˣ�
��3������ܼ�����Լ������ᣬ��������˾ͼ����ᣬ���ʹ��ǰ���ɵ�Mg��OH��2��BaCO3��CaCO3�ܽ⣬����ȥMg2+��Ba2+��Ca2+���ʴ�Ϊ������������ɵ�Mg��OH��2��BaCO3��CaCO3�ܽ⣬����ȥMg2+��Ba2+��Ca2+��
��4��Na2CO3����������Һ���й�����CO32-�������μ�Na2CO3��Һ�������ٲ�����������˵�����Լ��Ѿ�����������Ϊ��ȡ�����ϲ���Һ���Թ��У������μ�Na2CO3��Һ�������ٲ�����������˵�����Լ��Ѿ��������ʴ�Ϊ��ȡ�����ϲ���Һ���Թ��У������μ�Na2CO3��Һ��
���� ������Ҫ���������ʵķ����Լ����ε��ᴿ���漰�������Լ���ѡ��ͼ���˳���ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| �� ���� | I A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 1 | A | |||||||
| 2 | D | E | I | G | ||||
| 3 | B | C | F | H |
��2��AԪ���� IԪ���γɵĻ�����Ļ�ѧʽ��H2O��H2O2�����Ƕ��ǹ��ۻ����� ������ۻ���������ӻ��������
��3����B��C��F�У�ԭ�Ӱ뾶��С����P��
��4��D��E��Ԫ�ص�����������ˮ�����У����Խ�ǿ����HNO3��
��5��EΪ��̬�⻯��ΪNH3��ʵ������ȡ���Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��6���õ���ʽ��ʾB��H��ɻ�������γɹ���
 ��
�� | A�� | Al2��SO4��3=2Al3++3SO42- | B�� | HF=H++F- | ||
| C�� | H3PO4?3H++PO43- | D�� | NaHCO3=Na++H++CO32- |
| A�� | ����ͭ��Һ | B�� | �������� | ||
| C�� | ������Һ | D�� | FeCl3��Һ��NaOH��Һ�Ļ���� |
| A�� | 2NaHCO3�TNa2CO3+CO2��+H2O | B�� | MnO2+4 HCl��Ũ���TMnCl2+Cl2��+2 H2O | ||
| C�� | 2 H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2 H2O+O2�� | D�� | Na2CO3+CaCl2�TCaCO3��+2NaCl |
| A�� | ��ɢϵ�����ᡢ����ͭ������ | B�� | ����ʣ��ռ���ᡢ���� | ||
| C�� | �Σ��Ȼ��ơ���������̼��� | D�� | �ǵ���ʣ�ʯī��CO2������ |
| A�� | һ����˫�ؽṹ���ķ���ʵ�������������� | |
| B�� | �÷���ԭ�Ӽ䶼�Թ��ۼ������� | |
| C�� | ��������һ���µĻ����� | |
| D�� | �����ʵ���Է�������Ϊ2400 |
 NOx������β���е���Ҫ��Ⱦ��֮һ��
NOx������β���е���Ҫ��Ⱦ��֮һ��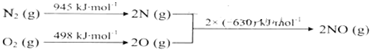
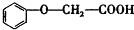 ����������Ӧ�IJ���A��һ��ʳ�����ϣ�����Ϊ��״�ṹ�Ҳ��������ش��������⣺
����������Ӧ�IJ���A��һ��ʳ�����ϣ�����Ϊ��״�ṹ�Ҳ��������ش��������⣺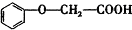 �ķ���ʽΪC8H8O3��
�ķ���ʽΪC8H8O3��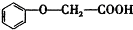 �ж���ͬ���칹�壬�����������࣬��ʹFeCl3��Һ����ɫ���ұ����ϵ�һ�ȴ��������ֵ�ͬ���칹��Ľṹ��ʽΪ
�ж���ͬ���칹�壬�����������࣬��ʹFeCl3��Һ����ɫ���ұ����ϵ�һ�ȴ��������ֵ�ͬ���칹��Ľṹ��ʽΪ ��
�� ��
�� ������һ�֣�����дһ�֣���
������һ�֣�����дһ�֣���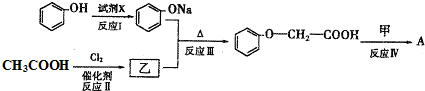
 ��
��