��Ŀ����
A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������B��Eͬ���壬C��FҲͬ���壬B��Cͬ���ڣ�A��B��ɵĻ������Ϊ��̬������A��Bԭ�Ӹ�����Ϊ4��1����A��C��ɵ����ֻ������������ΪҺ̬������A��Cԭ�Ӹ�����Ϊ1��1������A��Cԭ�Ӹ�����Ϊ2��1��һ��D+�����к���10�����ӣ�D��C������������������������Ϊ2��1������Է�������Ϊ78�Ļ����ﶡ���ش��������⣺
��1���ס����ĵ���ʽ���� ���� ��BC2�Ľṹʽ ��F���ӵĽṹʾ��ͼ ��
��2����ԭ�Ӱ뾶��D E���������������ͬ����
������������Ӧˮ��������ԣ�B E��
���ȶ����ʵ��۵㣺B C��
��3��BC2���� ���壬EC2���� ���壨�ԭ�ӡ����ӡ����ӡ���������
��4����A��C��D��ɵij����������к��еĻ�ѧ���� ��
��5���ҵ�ˮ��Һ��FC2��Ӧ�Ļ�ѧ����ʽ ��
��1���ס����ĵ���ʽ����
��2����ԭ�Ӱ뾶��D
������������Ӧˮ��������ԣ�B
���ȶ����ʵ��۵㣺B
��3��BC2����
��4����A��C��D��ɵij����������к��еĻ�ѧ����
��5���ҵ�ˮ��Һ��FC2��Ӧ�Ļ�ѧ����ʽ
������A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������D+�����к���10�����ӣ���DΪNaԪ�أ�D��C������������������������Ϊ2��1������Է�������Ϊ78�Ļ����ﶡ��C��ԭ������С����Ԫ�أ��ʻ����ﶡΪNa2O2��CΪ��Ԫ�أ�A��B��ɵĻ������Ϊ��̬��B��Cͬ���ڣ�����A��Bԭ�Ӹ�����Ϊ4��1���������ΪCH4��AΪ��Ԫ�ء�BΪ̼Ԫ�أ���A��C��ɵ����ֻ������������ΪҺ̬������A��Cԭ�Ӹ�����Ϊ1��1������ΪH2O2������A��Cԭ�Ӹ�����Ϊ2��1�����ΪH2O��B��Eͬ���壬��EΪSiԪ�أ�C��FҲͬ���壬��FΪ��Ԫ�أ��ݴ˽��
����⣺A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������D+�����к���10�����ӣ���DΪNaԪ�أ�D��C������������������������Ϊ2��1������Է�������Ϊ78�Ļ����ﶡ��C��ԭ������С����Ԫ�أ��ʻ����ﶡΪNa2O2��CΪ��Ԫ�أ�A��B��ɵĻ������Ϊ��̬��B��Cͬ���ڣ�����A��Bԭ�Ӹ�����Ϊ4��1���������ΪCH4��AΪ��Ԫ�ء�BΪ̼Ԫ�أ���A��C��ɵ����ֻ������������ΪҺ̬������A��Cԭ�Ӹ�����Ϊ1��1������ΪH2O2������A��Cԭ�Ӹ�����Ϊ2��1�����ΪH2O��B��Eͬ���壬��EΪSiԪ�أ�C��FҲͬ���壬��FΪ��Ԫ�أ�
��1����ΪCH4������ʽΪ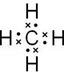 ����ΪNa2O2������ʽΪ
����ΪNa2O2������ʽΪ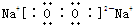 ��CO2�ĽṹʽΪ��O=C=O�������ӵĽṹʾ��ͼΪ
��CO2�ĽṹʽΪ��O=C=O�������ӵĽṹʾ��ͼΪ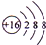 ��
��
�ʴ�Ϊ�� ��
��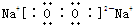 ��O=C=O��
��O=C=O�� ��
��
��2����ͬ�����������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��Na��Si��
�ʴ�Ϊ������
��ͬ�������϶��·ǽ����Լ������ǽ�����Խǿ��ۺ����������Խǿ��������H2CO3��H2SiO3��
�ʴ�Ϊ������
�۳����£�̼����Ϊ���壬����Ϊ���壬���ȶ����ʵ��۵㣺̼��������
�ʴ�Ϊ������
��3��CO2���ڷ��Ӿ��壬SiO2����ԭ�Ӿ��壬
�ʴ�Ϊ�����ӣ�ԭ�ӣ�
��4����A��C��D��ɵij���������ΪNaOH��NaOH�к������Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
��5��H2O2��ǿ�����ԣ���SO2��Ӧ�������ᣬ��Ӧ��ѧ����ʽΪ��H2O2+SO2=H2SO4��
�ʴ�Ϊ��H2O2+SO2=H2SO4��
��1����ΪCH4������ʽΪ
 ����ΪNa2O2������ʽΪ
����ΪNa2O2������ʽΪ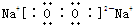 ��CO2�ĽṹʽΪ��O=C=O�������ӵĽṹʾ��ͼΪ
��CO2�ĽṹʽΪ��O=C=O�������ӵĽṹʾ��ͼΪ ��
���ʴ�Ϊ��
 ��
��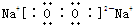 ��O=C=O��
��O=C=O�� ��
����2����ͬ�����������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��Na��Si��
�ʴ�Ϊ������
��ͬ�������϶��·ǽ����Լ������ǽ�����Խǿ��ۺ����������Խǿ��������H2CO3��H2SiO3��
�ʴ�Ϊ������
�۳����£�̼����Ϊ���壬����Ϊ���壬���ȶ����ʵ��۵㣺̼��������
�ʴ�Ϊ������
��3��CO2���ڷ��Ӿ��壬SiO2����ԭ�Ӿ��壬
�ʴ�Ϊ�����ӣ�ԭ�ӣ�
��4����A��C��D��ɵij���������ΪNaOH��NaOH�к������Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
��5��H2O2��ǿ�����ԣ���SO2��Ӧ�������ᣬ��Ӧ��ѧ����ʽΪ��H2O2+SO2=H2SO4��
�ʴ�Ϊ��H2O2+SO2=H2SO4��
���������⿼��ṹ����λ�ù�ϵ�����û�ѧ���Ԫ�������ɡ���ѧ�����������͵ȣ��Ѷ��еȣ��ƶ�Ԫ���������ǽ���Ĺؼ��������ﶡ���ƶ�Ϊ�״��㣬������Ϊ�����ƣ������Թ������ƣ�

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�