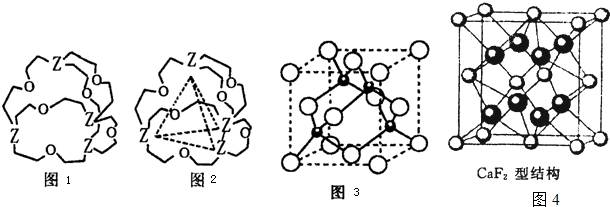
��Ŀ����
[��ѧ--ѡ�����ʽṹ������]
��֪A��B��C��D��E��F��Gλ��Ԫ�����ڱ���ǰ�����ڣ���Ԫ��ԭ�������������ӣ�A��ɫ��Ӧ�ʻ�ɫ����ҵ���õ��B�����ڵ��Ȼ������Ʊ�B��C��һ���ܱ�HF��NaOH��Һ�ܽ�ĵ��ʣ�D�ĵ縺�Ա��״�һ������ȴ����С��E�������Ʊ�Ư��Һ��ԭ�ϣ�F���γɺ�ɫ����ש��ɫ���ͺ�ɫ�����������G��һ�����������
��1��ǰ����������Ԫ���У���̬ԭ����δ�ɶԵ�������������������ͬ��Ԫ���� �֣�
��2��Ԫ��A��B��C�ֱ�����������γ�����X��Y��Z�۵���±���
���ͱ��з������۵�����ԭ�� ��
��3����֪���������£����Է���DOE2��һ��Һ̬���������ԭ��D���ӻ���ʽ�� ����ʢ��10mLˮ����ƿ�еμ�������DOE2��Һ�����������д̼�����ζ�����壮����д�˷�Ӧ�Ļ�ѧ����ʽ ��
��4��G�뵪ԭ�ӿ�1��1���ϣ��γ��˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ƣ�Gԭ�ӵļ۵����Ų�ʽΪ ���ڸúϳɲ����У���ͬһ��Gԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�������壮�����ֻ������������У��˾������� ���壮
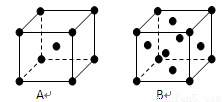
��5��F����Ķѻ���ʽ�� ����ѻ����ƣ�������λ��Ϊ �� ��F����������Һ�еμӰ�ˮֱ��������д���˹������漰���������ӷ���ʽ ���ݼ۲���ӶԻ������ۣ�Ԥ��SO42-�Ŀռ乹��Ϊ ��
��֪A��B��C��D��E��F��Gλ��Ԫ�����ڱ���ǰ�����ڣ���Ԫ��ԭ�������������ӣ�A��ɫ��Ӧ�ʻ�ɫ����ҵ���õ��B�����ڵ��Ȼ������Ʊ�B��C��һ���ܱ�HF��NaOH��Һ�ܽ�ĵ��ʣ�D�ĵ縺�Ա��״�һ������ȴ����С��E�������Ʊ�Ư��Һ��ԭ�ϣ�F���γɺ�ɫ����ש��ɫ���ͺ�ɫ�����������G��һ�����������
��1��ǰ����������Ԫ���У���̬ԭ����δ�ɶԵ�������������������ͬ��Ԫ����
��2��Ԫ��A��B��C�ֱ�����������γ�����X��Y��Z�۵���±���
| ������ | X | Y | Z |
| �۵�/K | 1266 | 1534 | 183 |
��3����֪���������£����Է���DOE2��һ��Һ̬���������ԭ��D���ӻ���ʽ��
��4��G�뵪ԭ�ӿ�1��1���ϣ��γ��˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ƣ�Gԭ�ӵļ۵����Ų�ʽΪ
��5��F����Ķѻ���ʽ��
������A��B��C��D��E��F��Gλ��Ԫ�����ڱ���ǰ�����ڣ���ԭ�������������ӣ�A��ɫ��Ӧ�ʻ�ɫ����AΪNa��C��һ���ܱ�HF��NaOH��Һ�ܽ�ĵ��ʣ���CΪSi��E�������Ʊ�Ư��Һ��ԭ�ϣ���EΪCl�����ԭ��������֪B��D���ڵ������ڣ���ҵ���õ��B�����ڵ��Ȼ������Ʊ�B��B���Ȼ���Ϊ���ӻ����Bԭ����������Na����BΪMg��D�ĵ縺�Ա��״�һ������ȴ����С����DΪSԪ�أ�F���γɺ�ɫ����ש��ɫ���ͺ�ɫ�������������FΪCu��G��һ�����������ԭ����������Cu���ɣ�4����G�뵪ԭ�ӿ�1��1���ϣ�G����+3�ۣ���Gֻ�ܴ��ڢ�A�壬��GΪGa���ݴ˽��
����⣺A��B��C��D��E��F��Gλ��Ԫ�����ڱ���ǰ�����ڣ���ԭ�������������ӣ�A��ɫ��Ӧ�ʻ�ɫ����AΪNa��C��һ���ܱ�HF��NaOH��Һ�ܽ�ĵ��ʣ���CΪSi��E�������Ʊ�Ư��Һ��ԭ�ϣ���EΪCl�����ԭ��������֪B��D���ڵ������ڣ���ҵ���õ��B�����ڵ��Ȼ������Ʊ�B��B���Ȼ���Ϊ���ӻ����Bԭ����������Na����BΪMg��D�ĵ縺�Ա��״�һ������ȴ����С����DΪSԪ�أ�F���γɺ�ɫ����ש��ɫ���ͺ�ɫ�������������FΪCu��G��һ�����������ԭ����������Cu���ɣ�4����G�뵪ԭ�ӿ�1��1���ϣ�G����+3�ۣ���Gֻ�ܴ��ڢ�A�壬��GΪGa��
��1����һ�����У���һ��δ�ɶԵ��ӵ�����ԭ�ӣ�������Ų�Ϊ1s1���ڶ������У�δ�ɶԵ�����������������C��1s22s22p2 �� O��1s22s22p4�����������У�δ�ɶԵ�������������P��1s22s22p63s23p3������������δ�ɶԵ������ĸ�����Fe��1s22s22p63s23p64s23d6����5�֣�
�ʴ�Ϊ��5��
��2��NaF�� MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2+�İ뾶��Na+�İ뾶С����MgF2���۵��NaF�ߣ�
�ʴ�Ϊ��NaF�� MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2+�İ뾶��Na+�İ뾶С����MgF2���۵��NaF�ߣ�
��3�����������£����Է���SOCl2��һ��Һ̬���������ԭ��Sԭ�ӵļ۲���Ӷ���=3+
=4������1�Թ¶Ե��ӣ���Sԭ�Ӳ�ȡsp3�ӻ�����ʢ��10mLˮ����ƿ�еμ�������SOCl2��Һ�����������д̼�����ζ�����壬Ӧ������SO2��HCl���÷�Ӧ�Ļ�ѧ����ʽSOCl2+H2O=SO2+2HCl��
�ʴ�Ϊ��sp3��SOCl2+H2O=SO2+2HCl��
��4��GΪGa�����ڵ������ڢ�A�壬��Gaԭ�Ӽ۵����Ų�ʽΪ4s24p1���ڸúϳɲ���GaN�У���ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�������壬GaN����ṹ�뵥�������ƣ������ֻ������������У��˾�������ԭ�Ӿ��壬
�ʴ�Ϊ��4s24p1��ԭ�ӣ�
��5��Cu���������������ܶѻ����Ե���Cuԭ��Ϊ�о�������֮�����Cuԭ�Ӵ��������ϣ�ÿ������Cuԭ��Ϊ12���湲�ã�������λ��Ϊ12�� ������ͭ��Һ�еμӰ�ˮֱ��������������������ͭ���������������ͭ�백ˮ����[Cu��NH3��4]2+���漰�ķ�Ӧ���ӷ���ʽΪ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O��
SO42-����ԭ��S�ļ۲���Ӷ�=4+
=4��Sԭ�ӹµ��Ӷ���Ϊ0����SO42-Ϊ��������ṹ��
�ʴ�Ϊ�������������ܶѻ���12��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O���������壮
��1����һ�����У���һ��δ�ɶԵ��ӵ�����ԭ�ӣ�������Ų�Ϊ1s1���ڶ������У�δ�ɶԵ�����������������C��1s22s22p2 �� O��1s22s22p4�����������У�δ�ɶԵ�������������P��1s22s22p63s23p3������������δ�ɶԵ������ĸ�����Fe��1s22s22p63s23p64s23d6����5�֣�
�ʴ�Ϊ��5��
��2��NaF�� MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2+�İ뾶��Na+�İ뾶С����MgF2���۵��NaF�ߣ�
�ʴ�Ϊ��NaF�� MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2+�İ뾶��Na+�İ뾶С����MgF2���۵��NaF�ߣ�
��3�����������£����Է���SOCl2��һ��Һ̬���������ԭ��Sԭ�ӵļ۲���Ӷ���=3+
| 6-2-1��2 |
| 2 |
�ʴ�Ϊ��sp3��SOCl2+H2O=SO2+2HCl��
��4��GΪGa�����ڵ������ڢ�A�壬��Gaԭ�Ӽ۵����Ų�ʽΪ4s24p1���ڸúϳɲ���GaN�У���ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�������壬GaN����ṹ�뵥�������ƣ������ֻ������������У��˾�������ԭ�Ӿ��壬
�ʴ�Ϊ��4s24p1��ԭ�ӣ�
��5��Cu���������������ܶѻ����Ե���Cuԭ��Ϊ�о�������֮�����Cuԭ�Ӵ��������ϣ�ÿ������Cuԭ��Ϊ12���湲�ã�������λ��Ϊ12�� ������ͭ��Һ�еμӰ�ˮֱ��������������������ͭ���������������ͭ�백ˮ����[Cu��NH3��4]2+���漰�ķ�Ӧ���ӷ���ʽΪ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O��
SO42-����ԭ��S�ļ۲���Ӷ�=4+
| 6+2-2��4 |
| 2 |
�ʴ�Ϊ�������������ܶѻ���12��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O���������壮
�����������ۺϿ������ʽṹ�����ʣ��漰Ԫ���ƶϡ�Ԫ�������ɡ���������Ų����������������ʡ��ӻ������۲���ӶԻ������ۡ����ӽṹ���������û�ѧ����ȣ�Ϊ�߿��������ͣ�������ѧ���ķ����������ۺ����û�ѧ֪ʶ�������Ŀ��飬��ȷ�ƶ�Ԫ���ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ