��Ŀ����
(1)��֪��
��Fe(s)��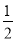 O2(g)=FeO(s)����H����272.0 kJ��mol��1
O2(g)=FeO(s)����H����272.0 kJ��mol��1
��2Al(s)��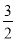 O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��____________________________________
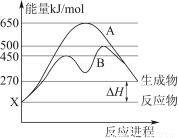
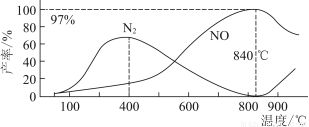
(2)ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B(����ͼ��ʾ)��
������ͼ�жϸ÷�Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����________(��������������С������������)��
������B���̱����˷�Ӧ���õ�����Ϊ________(ѡ�����)��
A�������¶ȡ������� B������Ӧ���Ũ�� C�������¶� D��ʹ�ô���
(3)1000 ��ʱ���������������������з�Ӧ��Na2SO4(s)��4H2(g) Na2S(s)��4H2O(g)
Na2S(s)��4H2O(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪ________________________________��
��֪K1000 ��<K1200 ������������ϵ�¶ȣ���������ƽ����Է�����������________(��������������С������������)��
(4)�����£����ȡ0.1 mol��L��1 HA��Һ��0.1 mol��L��1 NaOH��Һ��������(��Ϻ���Һ����ı仯���Բ���)����û��Һ��pH��8��
�����Һ����ˮ�������OH��Ũ����0.1 mol��L��1 NaOH��Һ����ˮ�������OH��Ũ��֮��Ϊ________��
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH________7(����<����>����������)����ͬ�¶��£������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ(�����)________��
a��NH4HCO3 b��NH4A c��(NH4)2CO3 d��NH4Cl
(1)3FeO(s)��2Al(s)=Al2O3(s)��3Fe(s)����H����859.7 kJ/mol
(2)��������D
(3)K��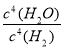 ����С
����С
(4)��107��������c��a��b��d
�������� (1)����ʽ�����١�3�ɵã�3FeO(s)��2Al(s)=Al2O3(s)��3Fe(s)���÷�Ӧ����H����1 675.7 kJ/mol��3��272.0 kJ/mol����859.7 kJ/mol��(2)�������Խ��ͷ�Ӧ�Ļ�ܣ�(3)�����Һ���Ũ�Ȳ�����ƽ�ⳣ������ʽ�У�ƽ�ⳣ��Խ����Ӧ���е�Խ��ȫ���÷�ӦΪ������������䵫�������ӵķ�Ӧ��������ϵ�¶ȣ�ƽ�ⳣ����С��˵��ƽ�����淴Ӧ�����ƶ���������������䵫������С���ʻ�������ƽ����Է����������С��(4)�����Һ��c(H��)��10��8 mol/L��c(OH��)��10��6 mol/L������OH��ȫ����ˮ���룻0.1 mol/L NaOH��Һ��c(OH��)��10��1 mol/L��c(H��)��10��13 mol/L������ˮ���������OH��Ũ�ȵ�����Һ��H��Ũ�ȣ�����Һ����ˮ�������OH��Ũ����0.1 mol��L��1 NaOH��Һ����ˮ�������OH��Ũ��֮��Ϊ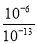 ��107����NH4AΪ���ԣ���֪A����NH4+ˮ��̶���ȣ���HA��Һ�ӵ�Na2CO3��Һ��������ų�����֪H2CO3���Ա�HA������HCO3��ˮ��̶ȱ�A���� ��CO32��ˮ��̶ȱ�HCO3����NH4HCO3��Һ��(NH4)2CO3��Һ���Լ����Ҽ���NH4HCO3��(NH4)2CO3��NH4ClΪǿ�������Σ�������
��107����NH4AΪ���ԣ���֪A����NH4+ˮ��̶���ȣ���HA��Һ�ӵ�Na2CO3��Һ��������ų�����֪H2CO3���Ա�HA������HCO3��ˮ��̶ȱ�A���� ��CO32��ˮ��̶ȱ�HCO3����NH4HCO3��Һ��(NH4)2CO3��Һ���Լ����Ҽ���NH4HCO3��(NH4)2CO3��NH4ClΪǿ�������Σ�������

�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | c(HA)/mol��L��1 | c(NaOH)/mol��L��1 | ��Ϻ���Һ��pH |
�� | 0.2 | 0.2 | pH��a |
�� | b | 0.2 | pH��7 |
�� | 0.2 | 0.1 | pH<7 |
�� | 0.1 | 0.1 | pH��c |
���жԻ�Ϻ���Һ���й�˵���У�����ȷ����(����)
A�����У���a��7����HA��ǿ��
B��������b��0.2����c(A��)��c(Na��)
C�����У���HA�����ᣬ��c(A��)>c(Na��)>c(H��)>c(OH��)
D��������c��9����c(OH��)��c(HA)��10��9 mol��L��1