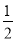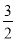��Ŀ����
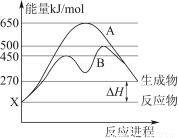
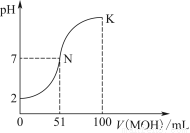
�����£���100 mL 0.01 mol��L��1 HA��Һ����μ���0.02 mol��L��1 MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(����仯���Բ���)��
�ش��������⣺
(1)��ͼ����Ϣ��֪HAΪ________��(����ǿ����������)��������________________________________________________��
(2)������һ��Ũ�ȵ�MAϡ��Һ��pH��a����a________________________________________________________7
(����>����<����������)�������ӷ���ʽ��ʾ��ԭ��Ϊ_____________________________________________________
��ʱ����Һ����ˮ�������c(OH��)��________��
(3)��д��K������Ӧ����Һ������Ũ�ȵĴ�С��ϵ��_________________________________________��
(4)K���Ӧ����Һ�У�c(M��)��c(MOH)________2c(A��)(����>����<����������)������ʱ��Һ�У�pH��10����c(M��)��c(OH��)��________mol��L��1��
(1)ǿ��0.01 mol��L��1 HA��pHΪ2��˵��HA��ȫ����
(2)<��M����H2O??MOH��H����1��10��a mol��L��1
(3)c(M��)>c(A��)>c(OH��)>c(H��)
(4)����0.005
��������(1)��ͼ��0.01 mol/L HA��Һ��pH��2��˵������ȫ���룬��Ϊǿ����ʡ�(2)����Ŀͼ���֪100 mL 0.01 mol/L HA��Һ�еμ�51 mL 0.02 mol/L MOH��Һ��pH��7��˵��MOH����������Ӧ��MA������ǿ���Σ�ˮ�������ԣ���Һ��H��ȫ����ˮ��������ģ���ˮ�������c(OH��)��1��10��a mol��L��1��(3)��(4)��K��ʱ��100 mL 0.01 mol/L HA��Һ�еμ�100 mL 0.02 mol/L MOH��Һ��Ӧ����Ӧ�����ҺΪ��Ũ�ȵ�MA��MOH��Һ����c(M��)>c(A��)>c(OH��)>c(H��)���������غ�ɵ�c(M��)��c(MOH)��2c(A��)����ϵ���غ㣺c(M��)��c(H��)��c(A��)��c(OH��)��c(M��)��c(OH��)��c(A��)��c(H��)��0.005 mol��L��1

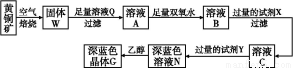
��ҵ���Ի�ͭ��(��Ҫ�ɷ���CuFeS2,���ʲ�����ˮ����)Ϊԭ��,�Ʊ���ɫ����G,�仯ѧʽΪ[Cu(NH3)4]SO4��H2O,�漰��������:
��֪25 ��ʱ,���ֽ�������������ܶȻ���������ȫ������pH��Χ���±�:
| Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
Ksp | 8.0��10-16 | 2.2��10-20 | 4.0��10-38 |
��ȫ����pH | ��9.6 | ��6.4 | ��3.2 |
(1)�ӿ��ͭ��������,�ɲ��õĴ�ʩ�� (д����)��
(2)����˫��ˮ���ܷ�����Ӧ�����ӷ���ʽΪ ;
�Լ�X�Ļ�ѧʽΪ������������������
(3)������,0.1 mol/L�Լ�Y��pH=11,����¶���,�Լ�Y�ĵ��볣��Ϊ��������,��pH��ֽ�����ҺpH�ķ����� ����
(4)��֪Cu(OH)2+4NH3��H2O [Cu(NH3)4]2++2OH-+4H2O,д���÷�Ӧ��ƽ�ⳣ������ʽ:������������������
[Cu(NH3)4]2++2OH-+4H2O,д���÷�Ӧ��ƽ�ⳣ������ʽ:������������������
(5)����ҺN�м����Ҵ���Ŀ���� ����
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
������ | CO32����SiO32����AlO2����Cl�� |
������ | Al3����Cu2����Mg2����NH4+��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y�����(V)�Ĺ�ϵ��ͼ��ʾ��
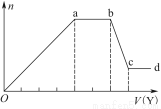
����Y�����ᣬ����Һ�к��еĽ�����������_________________________________��
ab�η�����Ӧ�������ӷ���ʽΪ___________________________________
����Oa����Y��Һ��Ӧ�����ӵ����ʵ���֮��Ϊ__________[Ҫ�������ӷ��ţ���n(Na��)]��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ______________________________
�����������ӵ�ˮ�����أ�����H����OH����Ӱ�죬����Һ��ֻ����4�����ӣ������ǵ����Ӹ�����Ϊ___________________________________[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
(2)��Ϊ����A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4����Sn=2Sn2����
2Sn2����O2��4H��=2Sn4����2H2O��
2H����SnO22�� Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH����
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�����ӷ���ʽ��______________________________________��
����������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������(����ʽ)__________��
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn(OH)2, �ü���__________��