��Ŀ����
����Ŀ���ȼ��仯�����������������Ӧ�ù㷺��
(1)��֪��900 Kʱ��4HCl(g)+O2(g)![]() 2Cl2(g)+2H2O(g)����Ӧ�Է���
2Cl2(g)+2H2O(g)����Ӧ�Է���
���÷�Ӧ�Ƿ��Ȼ������ȣ��жϲ�˵������______________________________________��
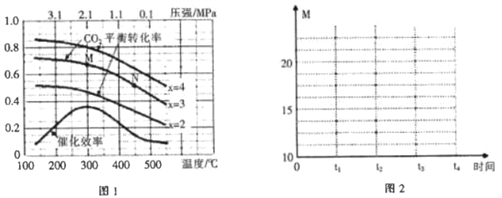
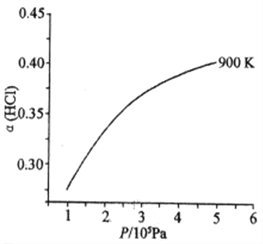
��900 Kʱ�������Ϊ4��l��HCl��O2�ں��º��ݵ��ܱ������з�����Ӧ��HCl��ƽ��ת������(HCl) ��ѹǿ(P)�仯������ͼ�����������������䣬���µ�T K(�ٶ���Ӧ���̲���)���뻭��ѹǿ��1.5��l05��4.5��105Pa��Χ�ڣ�HCl��ƽ��ת������(HCl)��ѹǿ(P)�仯����ʾ��ͼ_________��
(2)��֪��Cl2(g)+2NaOH(aq)==NaCl(aq)+NaClO(aq)+H2O(l) ��Hl=�D102 kJ��mol-1
3Cl2(g)+6NaOH(aq)==5NaCl(aq)+NaClO3(aq)+3H2O(1) ��H2=�D422 kJ��mol��1
��д������Һ��NaClO�ֽ�����NaClO3���Ȼ�ѧ����ʽ_____________________��
���ù�������NaOH��Һ�����������Ƶ�NaClO��Һ(����NaClO3)����ʱClO�D��Ũ��Ϊc0 mol��L-1������ʱNaClOת��ΪNaClO3�����tʱ����Һ��ClO�DŨ��Ϊct mol��L-1��д����ʱ����Һ��Cl�DŨ�ȵı���ʽ��c(Cl�D)=_________ mol��L-1 (��c0��ct��ʾ)
�����о�����������NaClO3�ķ�Ӧ���������У�
I��2ClO�D=ClO2�D+Cl�D
II��ClO2�D+ClO�D=ClO3�D+Cl�D
�����£���ӦII�ܿ��ٽ��У���������NaOH��Һ��Ӧ���ѵõ�NaClO3��������ײ���۽�����ԭ��_______________________________��
(3)���NaClO3ˮ��Һ���Ʊ�NaClO4���ڵ�����������������������������ʹ����ѹ���ߣ����Ч���½���Ϊ���������IJ�������ѡ����ʵ�����(����������)��д���õ����ܻ�ѧ����ʽ________________________________________��
���𰸡� ���ȣ���S��0����Ӧ�Է������H-T��S��0 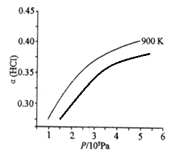 3NaClO��aq��=2NaCl��aq��+NaClO3��aq����H=-116kJ/mol (5c0-2ct)/3 ��Ӧ��Ļ�ܸߣ�����Ӱٷ����ͣ�������ClO-��ClO3-ת�� 2NaClO3+O2
3NaClO��aq��=2NaCl��aq��+NaClO3��aq����H=-116kJ/mol (5c0-2ct)/3 ��Ӧ��Ļ�ܸߣ�����Ӱٷ����ͣ�������ClO-��ClO3-ת�� 2NaClO3+O2![]() 2NaClO4
2NaClO4
����������1���ٷ�Ӧ�Է����е��ж������ǡ�H-T��S��0��
�ڸ����¶ȡ�ѹǿ��ƽ��״̬��Ӱ������жϣ�
��2���ٸ�˹���ɼ���NaClO�ֽ�����NaClO3���Ȼ�ѧ����ʽ��
�������������������������ӣ��������Ʒֽ����������ӣ���Һ��������Ϊ�����ܺͣ�
�۸��ݻ�ܶԷ�Ӧ��Ӱ�������
��3��Ϊ���������IJ�������ѡ����ʵ�����������������Ӧ����ˮ��
��1����900 ��ʱ��4HCl(g)+O2(g)![]() 2Cl2(g)+2H2O(g)�ġ�S��0����Ӧ�Է������H-T��S��0����H��0��
2Cl2(g)+2H2O(g)�ġ�S��0����Ӧ�Է������H-T��S��0����H��0��
��900Kʱ�������Ϊ4��1��HCl��O2�ں��º��ݵ��ܱ������з�����Ӧ��HCl��ƽ��ת��������HCl����ѹǿ��P���仯������ͼ�����������������䣬���µ�T K��ƽ��������У�HClת���ʼ�С����ѹǿ����ƽ��������У�HClת�������ݴ˻���ͼ��Ϊ ��
��
��2������֪��
��Cl2(g)+2NaOH(aq)==NaCl(aq)+NaClO(aq)+H2O(l) ��Hl=�D102 kJ��mol-1
��3Cl2(g)+6NaOH(aq)==5NaCl(aq)+NaClO3(aq)+3H2O(1) ��H2=�D422 kJ��mol��1
���ݸ�˹���ɿ�֪��-���3�õ�NaClO�ֽ�����NaClO3���Ȼ�ѧ����ʽ��3NaClO��aq��=2NaCl��aq��+NaClO3��aq����H=-116kJ/mol��
���ù�������NaOH��Һ�����������Ƶ�NaClO��Һ������NaClO3������ʱClO-��Ũ��Ϊc0 molL-1����Cl2��g��+2NaOH��aq��=NaCl��aq��+NaClO��aq��+H2O��l����Ӧ������������Ũ��Ϊc0 molL-1������ʱNaClOת��ΪNaClO3�����tʱ����Һ��ClO-Ũ��Ϊct mol��L-1����Ӧ�Ĵ�������Ũ��=c0 molL-1-ct mol��L-1
3NaClO��aq��=NaClO3��aq��+2NaCl��aq��
3 2
c0 molL-1-ct mol��L-1 2(c0 molL-1-ct mol��L-1)/3
��ʱ����Һ��Cl-Ũ�ȵı���ʽ��c0 molL-1+2(c0 molL-1-ct mol��L-1)/3=(5c0-2ct)/3 mol
�۳����£���ӦII�ܿ��ٽ��У���������NaOH��Һ��Ӧ���ѵõ�NaClO3��˵����Ӧ���о����ڷ�Ӧ��Ӧ��Ļ�ܸߣ�����Ӱٷ����ͣ�������ClO-��ClO3-ת����
��3�����NaClO3ˮ��Һ���Ʊ�NaClO4���ڵ�����������������������������ʹ����ѹ���ߣ����Ч���½���Ϊ���������IJ�������ѡ����ʵ�����������������Ӧ����ˮ���õ����е��Ļ�ѧ����ʽΪ��2NaClO3+O2![]() 2NaClO4��
2NaClO4��

����Ŀ��ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr��OH��3��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2��O2
��1���÷�Ӧ�еĻ�ԭ����____________��
��2���÷�Ӧ�У�������ԭ��Ӧ�Ĺ�����______________��____________��
��3��д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ_____________��
��4���練Ӧת����0.3 mol���ӣ�������������ڱ�״���µ����Ϊ ��
��5����֪I-��Fe2+��SO2��Cl-��H2O2���л�ԭ�ԣ�������������Һ�л�ԭ�Ե�ǿ��˳��Ϊ��SO2>I->H2O2>Fe2+>Cl-�������з�Ӧ���ܷ������ǣ� ��
A��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+ |
B��I2+SO2+2H2O=H2SO4+2HI |
C��H2O2+H2SO4=SO2��+O2��+2H2O |
D��2Fe3++2I-=2Fe2++I2 |