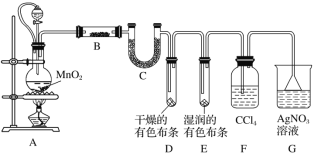
��Ŀ����
����Ŀ��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ�����������������������������գ�
��1���������������������漰���Ļ�ѧ�������Ԫ���У����ڵ�������Ԫ�ص���___��д��N�ĺ�������Ų�ʽ___��
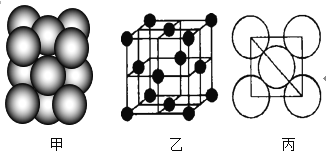
��2����֪SO2���ӵĿռ乹��Ϊ�����Σ���SO2Ϊ___��ѡ���������������Ǽ����������ӡ�
��3��������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ�ʺ����Һ������Ũ�ȵ��й��������£��������Ӻ��Բ��ƣ���
���� | Na+ | SO42- | NO3- | OH- | Cl- |
Ũ��/��mol��L-1�� | 5.5��10-3 | 8.5��10-4 | y | 2.0��10-4 | 3.4��10-3 |
�ٷ�Ӧ����ҺpH___7������y=___mol��L-1��
��д��NaClO2��Һ����SO2�����ӷ���ʽ___��
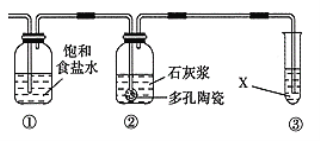
��4�������е�SO2���ɲ��ð��������ȥ���䷴Ӧԭ��������ͼ��ʾ��
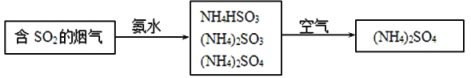
��д��SO2����ˮ��Ӧ����NH4HSO3�Ļ�ѧ����ʽ___��
��(NH4)2SO4��Һ��Ũ������������___��
���𰸡�Na��S��Cl 1s22s22p3 ���� �� 2.0��10-4mol��L-1 ClO2-+2SO2+4OH-��2SO42-+Cl-+2H2O SO2+NH3��H2O��NH4HSO3 NH4+
��������
��1��Nԭ�Ӻ�����7�����ӣ������������ԭ���Ų���
��2��SO2���ӵĿռ乹��Ϊ�����Σ�����������ɵ����IJ��غϣ�
��3���ٸ��ݷ�Ӧ����Һ�������ӵ�Ũ���ж�pH�����ݵ���ؼ���yֵ��
��SO2��NaClO2��Һ����ΪSO42-��NaClO2����ԭΪCl-��
��4����SO2����ˮ1:1��Ӧ����NH4HSO3��
��(NH4)2SO4��ǿ����ʣ���ȫ���룬 c(NH4+)�ӽ� c(SO42-)�Ķ�����
��1������Ԫ�����ڱ������ڵ�������Ԫ�ص���Na��S��Cl��Nԭ�Ӻ�����7�����ӣ���������Ų�ʽ��1s22s22p3��
��2��SO2���ӵĿռ乹��Ϊ�����Σ�����������ɵ����IJ��غϣ�SO2Ϊ���Է��ӣ�
��3���ٷ�Ӧ����Һ�����������ӵ�Ũ����2.0��10-4��������Ũ����5.0��10-11��������Ũ��С�����������ӵ�Ũ�ȣ�������Һ�ʼ��ԣ�pH��7�����ݵ���غ�5.5��10-3+5.0��10-11=(8.5��10-4)��2+y+2.0��10-4+3.4��10-3�����y=2.0��10-4mol��L-1��
��SO2��NaClO2��Һ����ΪSO42-��NaClO2����ԭΪCl-����Ӧ�����ӷ���ʽ��ClO2-+2SO2+4OH-��2SO42-+Cl-+2H2O��
��4����SO2����ˮ1:1��Ӧ����NH4HSO3����Ӧ�Ļ�ѧ����ʽ��SO2+NH3��H2O��NH4HSO3��
��(NH4)2SO4��ǿ����ʣ���ȫ���룬 c(NH4+)�ӽ� c(SO42-)�Ķ���������Ũ������������NH4+��