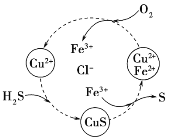
��Ŀ����
����Ŀ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ_______________��
(2)YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ_____________��
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ________��
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��___________��
(5)�Ƚ�X���⻯����ͬ��ڶ�����������Ԫ�����γɵ��⻯��е�ߵ�______��
���𰸡�1s22s22p63s23p63d104s24p3 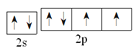 ������ As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O NH3>AsH3>PH3
������ As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O NH3>AsH3>PH3
��������
XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�XԪ��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3�����ڵ������ڵ�VA�壬��XΪAsԪ�أ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ�Y��2p�������2�����ӻ�4�����ӣ�����YΪ̼Ԫ�ػ���Ԫ�أ�X��Y���γɻ�����X2Y3����YΪOԪ�أ�X��Y��Z����Ԫ�ص�ԭ������֮�͵���42����Z��������Ϊ42-8-33=1����ZΪHԪ�أ���ԭ�ӿ����γɸ�һ�����ӣ��������⣬Ȼ����һ�������
��������������֪��X��AsԪ�أ�Y��OԪ�أ�Z��HԪ�ء�
(1)X ��AsԪ�أ�XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ��1s22s22p63s23p63d104s24p3��
(2)Y��OԪ�أ���������Ų�ʽΪ1s22s22p4���۲�����Ų�ʽ��2s22p4����������ʾʽΪ�� ��
��
(3)X��As��Z��H��As��H����Ԫ���Թ��ۼ��γ�AsH3������As��NΪͬ����Ԫ�أ�AsH3�ռ�ṹ��NH3���ƣ��������ͣ�
(4)X��As��Y��O�������γ�As2O3��Zn��H2SO4��Ӧ����AsH3��ZnSO4��H2O������ԭ���غ㡢�����غ㣬�ɵ÷�Ӧ����ʽΪ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
(5)As�ǵ������ڵ�VA��Ԫ�أ��������е�VA��Ԫ�ػ���N��P�������γɵ������ֱ���AsH3��NH3��PH3�����������Ϊ���Ӿ��壬����֮��ͨ�����Ӽ���������ϣ����ʵ���Է�������Խ���Ӽ�������Խ�����ʵ��۷е��Խ�ߡ�������NH3�ķ��Ӽ��������������˷���֮�����������ʹNH3�ķе���ߣ����������ʷе��ɸߵ��͵�˳���ǣ�NH3>AsH3>PH3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ�����������������������������գ�
��1���������������������漰���Ļ�ѧ�������Ԫ���У����ڵ�������Ԫ�ص���___��д��N�ĺ�������Ų�ʽ___��
��2����֪SO2���ӵĿռ乹��Ϊ�����Σ���SO2Ϊ___��ѡ���������������Ǽ����������ӡ�
��3��������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ�ʺ����Һ������Ũ�ȵ��й��������£��������Ӻ��Բ��ƣ���
���� | Na+ | SO42- | NO3- | OH- | Cl- |
Ũ��/��mol��L-1�� | 5.5��10-3 | 8.5��10-4 | y | 2.0��10-4 | 3.4��10-3 |
�ٷ�Ӧ����ҺpH___7������y=___mol��L-1��
��д��NaClO2��Һ����SO2�����ӷ���ʽ___��
��4�������е�SO2���ɲ��ð��������ȥ���䷴Ӧԭ��������ͼ��ʾ��
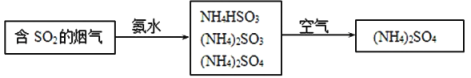
��д��SO2����ˮ��Ӧ����NH4HSO3�Ļ�ѧ����ʽ___��
��(NH4)2SO4��Һ��Ũ������������___��