��Ŀ����
14��A��B��C��D��E��Ϊ�л������֮��Ĺ�ϵ��ͼ��ʾ��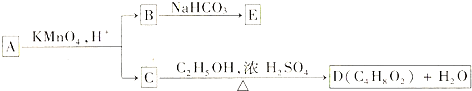
��֪RCH�TCHR�����Ը��������Һ�з�Ӧ����RCOOH��R��COOH������R��R��Ϊ�������ش��������⣺
��1��ֱ��������A����C��H��O����Ԫ�أ�����Է�������С��90����������Ԫ�ص���������Ϊ0.186����A�ķ���ʽΪC5H10O��
��2����֪B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������Ũ����Ĵ��£�B��������C2H5OH������Ӧ�Ļ�ѧ����ʽ��HOOCCH2COOH+2C2H5OH$��_{��}^{Ũ����}$C2H5OOCCH2COOC2H5+2H2O��
��3��A��������������÷ų���������ʹ������Ȼ�̼��Һ��ɫ����A�Ľṹ��ʽ��HOCH2CH2CH=CHCH3��
��4��D��ͬ���칹���У�����NaHCO3��Һ��Ӧ�ų�CO2����2�֣�
���� ������������Ϊ0.186���ٶ�A����Է�������Ϊ90����N��O��=$\frac{90��0.186}{16}$=1.0463����������ԭ�Ӹ���Ϊ1����A����Է�������Ϊ��$\frac{16}{0.186}$=86�������෨��$\frac{86-16}{12}$=5��10������A�÷���ʽΪC5H10O�����C+C2H5OH$��_{��}^{Ũ����}$C4H8O2+H2O��֪��CΪCH3COOH��DΪCH3COOC2H5������B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������B�к���2��-COOH��B�к���3��Cԭ�ӣ�����֪B�к���2��-COOH����B�л�����һ��CH2������B�Ľṹ��ʽΪHOOCCH2COOH���л���EΪNaOOCCH2COONa��A��������������÷ų�������˵��A�к��еĹ�����Ϊ-OH����ʹ������Ȼ�̼��Һ��ɫ��˵��A�к���C=C����AΪֱ������������Ŀ��Ϣ��B��C�ṹ���ɵ�A�Ľṹ��ʽΪ��HOCH2CH2CH=CHCH3���ݴ˽��
��� �⣺��1��������������Ϊ0.186���ٶ�A����Է�������Ϊ90����N��O��=$\frac{90��0.186}{16}$=1.0463����������ԭ�Ӹ���Ϊ1����A����Է�������Ϊ��$\frac{16}{0.186}$=86�������෨��$\frac{86-16}{12}$=5��10������A�÷���ʽΪC5H10O��
�ʴ�Ϊ��C5H10O��
��2�����C+C2H5OH$��_{��}^{Ũ����}$C4H8O2+H2O��֪��CΪCH3COOH��DΪCH3COOC2H5������B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������B�к���2��-COOH��B�к���3��Cԭ�ӣ�����֪B�к���2��-COOH����B�л�����һ��CH2������B�Ľṹ��ʽΪHOOCCH2COOH��B��������C2H5OH��Ӧ�Ļ�ѧ����ʽΪ��HOOCCH2COOH+2C2H5OH$��_{��}^{Ũ����}$C2H5OOCCH2COOC2H5+2H2O��
�ʴ�Ϊ��HOOCCH2COOH+2C2H5OH$��_{��}^{Ũ����}$C2H5OOCCH2COOC2H5+2H2O��
��3��A��������������÷ų�������˵��A�к��еĹ�����Ϊ-OH����ʹ������Ȼ�̼��Һ��ɫ��˵��A�к���C=C����AΪֱ������������Ŀ��Ϣ��B��C�ṹ���ɵ�A�Ľṹ��ʽΪ��HOCH2CH2CH=CHCH3��
�ʴ�Ϊ��HOCH2CH2CH=CHCH3��
��4��DΪCH3COOC2H5����ͬ���칹������NaHCO3��Һ��Ӧ�ų�CO2��˵��Ϊ���ᣬ��ṹ��ʽΪ��CH3CH2CH2COOH��CH3CH��CH3��COOH��
�ʴ�Ϊ��2��
���� ���⿼���л�����ƶϣ��ؼ��Ǽ���ȷ��A�ķ���ʽ���ٽ����Ŀ��Ϣ����Ӧ������D�ķ���ʽ�����ƶϣ����ؿ���ѧ�����������������Ѷ��еȣ�

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�| A�� | 1molNa2O2�����������ӵĸ���Ϊ2NA | |
| B�� | ��״���£�2.24L�������ķ�������Ϊ0.1NA | |
| C�� | 32gO2��32g O3����������ԭ��������Ϊ2NA | |
| D�� | 6.4gͭ�������Ũ������ȫ��Ӧת�Ƶĵ�������0.1NA |
| A�� | XQ2W�����ڳ����³���̬�������е��ĸ�ԭ����ͬһƽ���� | |
| B�� | Z��W�γɵ��������ӻ����������������Ӹ����Ⱦ�Ϊ2��1 | |
| C�� | X��W��Ԫ�طֱ��QԪ�ػ��ϣ����γɵ�������ͬ�����ֻ����� | |
| D�� | ��Ӧ�����Ӱ뾶��С��ϵΪ��Y��W��Z |
| A�� | pH=1����Һ�У�Na+��NH4+��SO42-��ClO- | |
| B�� | KW/c��H+��=0.1 mol•L-1����Һ�У�Na+��K+��MnO4-��HCO3- | |
| C�� | 0.1 mol•L-1��Na2SO3��Һ�У�K+��H+��SO42-��NO3- | |
| D�� | ����������Һ�У�Fe3+��Na+��Cl-��SO42- |
| A�� | ���ԣ�HClO4��H2SO4��H3AsO4��H3PO4 | |
| B�� | ���ԣ�Ca��OH��2��Mg��OH��2��Al��OH��3 | |
| C�� | ���������û������������Cs��Rb��K��Ca | |
| D�� | �����ԣ�F2��Cl2��S |
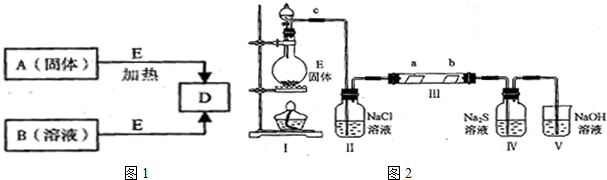
 ��
��
 ��֪�飨As��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
��֪�飨As��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ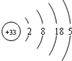
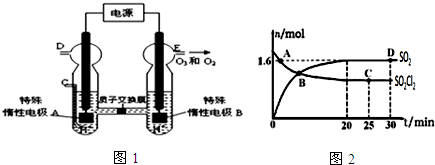
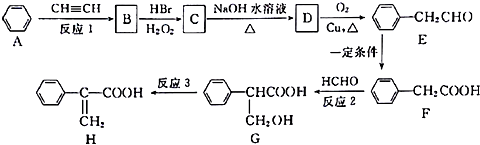
 ��
�� +NaOH $��_{��}^{ˮ}$
+NaOH $��_{��}^{ˮ}$ +NaBr��
+NaBr�� ��
��