��Ŀ����
18��������A��B����������ɵĻ�����壨A����Է�������С��B����Է����������������������������ֻ���е���������Ԫ�أ����Ҳ���A��B�Ժ��ֱ�����ϣ���������������ܴ���$\frac{14}{3}$���ɴ˿�ȷ��AΪNH3���������������ĵ������������Ϊ7��1�����ڻ��������A��B�����ʵ���֮��Ϊ4��1��A�ڻ�������е��������Ϊ80%������״���µ�a L��A����1000gˮ�У��õ�����Һ�ܶ�Ϊb g/mL������Һ���ʵ���Ũ��Ϊ$\frac{1000ab}{22400+17a}$mol/L������ ��NH3 �е������������Ϊ$\frac{14}{3}$���ڴ�NH3 �л����κα�����N2 ������ʹ������������ȴ���$\frac{14}{3}$����ΪN2 ��NH3�Ļ�����壬A����Է�������С��B����Է�����������AΪNH3��BΪN2 ���ݴ˼�����
��� �⣺��NH3 �е������������Ϊ$\frac{14}{3}$���ڴ�NH3 �л����κα�����N2 ������ʹ������������ȴ���$\frac{14}{3}$����ΪN2 ��NH3�Ļ�����壬A����Է�������С��B����Է�����������AΪNH3��BΪN2 ��
�������������ĵ������������Ϊ7��1���谱��Ϊxmol������Ϊymol����x+2y����14��3x=7��1��������x��y=4��1��
A�ڻ�������е��������Ϊ$\frac{4}{5}$��100%=80%��
����״���£�a LNH3�����ʵ���Ϊ$\frac{aL}{22.4L/mol}$=$\frac{a}{22.4}$mol����������Ϊ$\frac{17a}{22.4}$g������Һ���Ϊ$\frac{��1000+\frac{17a}{22.4}��g}{1000bg/L}$=$\frac{22400+17a}{22400b}$L������Һ���ʵ���Ũ��Ϊ$\frac{\frac{a}{22.4}mol}{\frac{22400+17a}{22400b}L}$=$\frac{1000ab}{22400+17a}$mol/L��
�ʴ�Ϊ��NH3 ��4��1��80%��$\frac{1000ab}{22400+17a}$��
���� ���⿼��������йؼ��㣬������ѧ���ķ��������ͼ��������Ŀ��飬�ؼ����ƶ����ʣ��Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� �״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��CO��g��+2H2��g��?CH3OH��g����H1=-116kJ•mol-1
�״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��CO��g��+2H2��g��?CH3OH��g����H1=-116kJ•mol-1����֪��2CO��g��+O2��g���T2CO2��g����H2=-566kJ•mol-1��2H2��g��+O2��g���T2H2O��g����H3=-484kJ•mol-1�����ʾ1mol��̬�״���ȫȼ������CO2��ˮ����ʱ���Ȼ�ѧ����ʽΪCH3OH��g��+3/2O2��g��=CO2��g��+2H2O��g����H=-651kJ•mol-1��
�����ݻ�Ϊ1L�ĺ��������У��ֱ��о���230�桢250�桢270�������¶��ºϳɼ״��Ĺ��ɣ���ͼ�����������¶��²�ͬ��H2��CO����ʼ��ɱȣ���ʼʱCO�����ʵ�����Ϊ1mol����COƽ��ת���ʵĹ�ϵ����ش�
A�������������¶��У�����Z��Ӧ���¶���270�森
B������ͼ��a���Ӧ�����ݣ����������Z�ڶ�Ӧ�¶���CO��g��+2H2��g��?CH3OH��g����ƽ�ⳣ��K=4L2•mol-2����д����λ��
����ij�¶��£���һ������CO��H2Ͷ��10L���ܱ������У�5minʱ�ﵽƽ�⣬�����ʵ����ʵ�Ũ�ȣ�mol•L-1���仯���±���ʾ��
| 0min | 5min | 10min | |
| CO | 0.1 | 0.05 | |
| H2 | 0.2 | 0.2 | |
| CH3OH | 0 | 0.04 | 0.05 |
| A�� | ����NaOH��Һ�����Ϊ0.5L | |
| B�� | ������������һ����4.48L | |
| C�� | Cu��Cu2O�����ᷴӦ��ʣ��Ϊ0.5mol | |
| D�� | Cu��Cu2O�����ʵ���֮����1��2 |
| �� | �� | �� | ���������� | ���������� | |
| A | Na2CO3 | H2SO4 | NaOH | SO2 | CO2 |
| B | NaOH | HCl | NaCl | Na2O | CO2 |
| C | NaOH | CH3COOH | CaF2 | CO | SO2 |
| D | KOH | HNO3 | CaCO3 | CaO | SO2 |
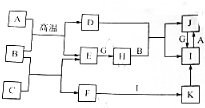 �й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����A��C��E��G����ѧ��ѧ�еij������ʣ�����G�ʻ���ɫ��E��G��ȼ�շ�����ɫ���棬F����ɫ��Ӧ�Ի�ɫ��B�dz�������ɫҺ�壬KΪ���ɫ��������ش��������⣺
�й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����A��C��E��G����ѧ��ѧ�еij������ʣ�����G�ʻ���ɫ��E��G��ȼ�շ�����ɫ���棬F����ɫ��Ӧ�Ի�ɫ��B�dz�������ɫҺ�壬KΪ���ɫ��������ش��������⣺ ��
��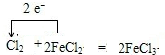 ��
�� ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����