��Ŀ����
15��ClO2��һ�ָ�Ч�����ס���ȫ��ɱ������������������ˮ���Ʊ��������£���1��������ʳ��ˮ�Ʊ������ƣ����ڵ���ʳ��ˮ���ȳ�ȥ���е�Ca2+��Mg2+��SO42-�����ʣ��ڳ��Ӳ���ʱ��������ˮ���ȼ��������BaCl2���ѧʽ�������������ٲ������ټ��������Na2CO3��NaOH����ַ�Ӧ����һ����ȥ��
��2������������õ���ʳ��ˮ���ض������µ��õ������ƣ�NaClO3�����ٽ��������ᷴӦ����ClO2��Cl2��ClO2��Cl2�����ʵ�������2��1��
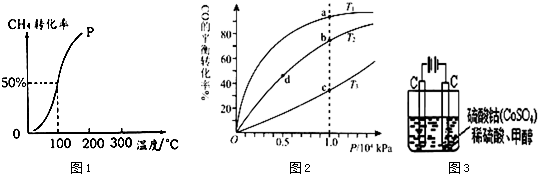
��3��ѧ������ͼ1��ʾװ��ģ�ҵ��ȡ���ռ�ClO2����NaClO3�Ͳ��ᣨH2C2O4��������60��ʱ��Ӧ�Ƶã�

��Ӧ��������Ҫ��A�������м��ȣ����ȵķ�ʽΪˮԡ���ȣ�������Ҫ�IJ����������ƾ����⣬�����¶ȼơ����ձ���
��4����Ӧ����װ��C�пɵ��������ƣ�NaClO2����Һ����֪NaClO2������Һ���¶ȵ���38��ʱ�������ľ�����NaClO2•3H2O�����¶ȸ���38��ʱ��������NaClO2������ͼ2��ʾNaClO2���ܽ�����ߣ�����ɴ�NaClO2��Һ���Ƶ�NaClO2•3H2O�IJ������裺
������Ũ��������ȴ������38�棩�ᾧ����ϴ�ӣ��ܸ��
��5��Ŀǰ�ҹ��ѳɹ����Ƴ�����NaClO2��ȡ�������ȵ��·�������Cl2ͨ�뵽NaClO2��Һ�У�����ȡ270kg�������ȣ���Ҫ�������Ƶ�������362kg��
��6��ClO2��Cl2���ܽ���Ʒ�ˮ�еľ綾CN-����Ϊ�����ʣ���������ԭΪCl-��������CN-��ͬ���ĵ�Ʒ�ˮ������Cl2�����ʵ�����ClO2��2.5����
���� ��1������Ҫ�����������ȥ�������������ƺ�̼���ƣ�Ҫ�ȼӹ������Ȼ�����ȥ��������ӣ�Ȼ����̼����ȥ�������ı�����
��2������NaClO3�����ᷴӦ����ClO2��Cl2�ķ�Ӧ��֪������ÿĦ��Cl2ʧȥ2mol���ӣ�������ÿĦ��ClO2�õ�1mol���ӣ����ݵ��ӵ�ʧ�غ���㣻
��3����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶ�ClO2��K2CO3��CO2�����Է�ӦҪ��ˮԡ���ȣ�
��4������Һ����ȡ���ʣ�һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����
��5�����ݷ�Ӧ����ʽ���м��㣻
��6��������CN-��ͬ���ĵ�Ʒ�ˮʱ��ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ���Ϊ2.5����
��� �⣺��1��Ҫ�ȳ���������ӣ�Ȼ���ٳ������ӣ�̼���ƿ��Գ�ȥ�����ı����ӣ�����ӷ��ˣ������ı����Ӿ�û����ȥ�����ڼ��������Ƴ�ȥþ����˳�������ƣ���Ϊ�������������Ƽ�����Ϳ��Ե����ˣ�ֻҪ���������ӳ����ˣ����˾����ˣ����������ȥ����������������̼������ӣ���γ��Ӳ���ʱ��������ˮ���ȼ��������BaCl2�����������ٲ������ټ��������Na2CO3����NaOH��Һ����ַ�Ӧ����һ����ȥ��
�ʴ�Ϊ��BaCl2��Na2CO3��
��2������NaClO3�����ᷴӦ����ClO2��Cl2�ķ�Ӧ��֪������ÿĦ��Cl2ʧȥ2mol���ӣ�������ÿĦ��ClO2�õ�1mol���ӣ����ݵ��ӵ�ʧ�غ��֪������ClO2��Cl2�����ʵ�������2��1��
�ʴ�Ϊ��2��1��
��3����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶ�ClO2��K2CO3��CO2�����Է�ӦҪ��ˮԡ���ȣ�Ϊ��ȷ����ˮԡ���¶���Ҫ���¶ȼƣ�����ˮԡװ���г��ƾ����⣬����Ҫ�ô��ձ���
�ʴ�Ϊ��ˮԡ���ȣ��¶ȼơ����ձ���
��4������Һ����ȡ���ʣ�һ���������Ũ������ȴ������38�棩�ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO2•3H2O�����ȹ��ˣ���38�桫60����ˮϴ�ӣ�����60����
�ʴ�Ϊ������Ũ���� ��ȴ������38�棩�ᾧ��
��5�����������Ƶ�������Ϊxkg��
���ݷ�Ӧ 2NaClO2+Cl2=2NaCl+2ClO2
181 135
x 270kg
����x=$\frac{270kg��181}{135}$=362
�ʴ�Ϊ��362kg��
��6��������CN-��ͬ���ĵ�Ʒ�ˮʱ��ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ�������Cl2�����ʵ�����ClO2��2.5����
�ʴ�Ϊ��2.5��
���� ���⿼�黯ѧ�������̣��漰������ԭ��Ӧ����ת�Ƽ��㣬��ѧʵ��������������ӣ������֪ʶ����Ŀ�Ѷ��еȣ�ע��������ʵķ��롢�ᴿ������������Ŀ�ѶȲ��ò��������Ǹ߿����ȵ㣬���ջ����ǹؼ���

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�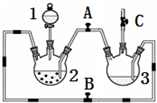 ij��ѧ��ȤС������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ����м��ϡ���ᡢ����������Һ��
ij��ѧ��ȤС������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ����м��ϡ���ᡢ����������Һ����1��ϡ����Ӧ����1�У���д�������ƣ���
��2����ʵ��ͨ������A��B��C�������أ��������еĿ����ž����ٹرտ���B������AC�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п��������ɷ�ֹ���ɵ�����������������
��3����FeSO4��Һ�м��루NH4��2SO4������Ʊ�Ħ���ξ���[��NH4��2SO4•FeSO4•6H2O]����Է�������392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ�ӣ�NH4��2SO4•FeSO4•6H2O�ֲ�Ʒ�����з���������ʵ���D
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol•L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ��յ�����������һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ
ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ$\frac{980c}{a}$��100%������ĸac��ʾ����
�ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������BC
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ�