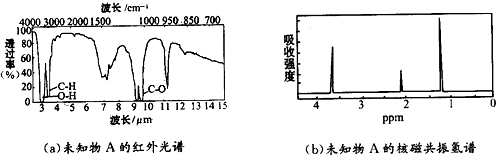
��Ŀ����
9��ij��ȤС��ͬѧ��ʵ�����ü���l-������ŨH2SO4���廯�ƻ����ķ������Ʊ�1-�嶡�飬�������ͼ��ʾ��ʵ��װ�ã����еļг�����û�л�������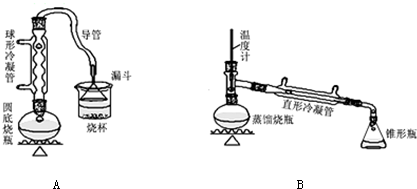
��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���Ǽȿ������ճ�֣��ֿ��Է�ֹ������
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ����ab��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С�-CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���𣺲�����������1-�嶡��Ҳ����-CH2CH2CH2CH3��
��4��Ϊ�˽�һ���ᴿ1-�嶡�飬��С��ͬѧ�������л�����й��������±���
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
���� ��1�����ݷ�������ԭ�����
��2��l-������Ũ����������£��ɷ������Ӽ�ͷ�������ˮ��
��3�����ݺ�������ǵ����ã����з��ӽṹ�ͻ�ѧ��ɷ�����
��4������1-�嶡��ķе���
��� �⣺��1������һ��©�����������Һ��ĽӴ�����������ܳ�ֱ������ҷ�ֹ������
�ʴ�Ϊ���ȿ������ճ�֣��ֿ��Է�ֹ������
��2��l-������Ũ����Ĵ������·�����������ˮ��ȡ��ϩ�����Ӽ���ˮ�õ��ѣ��������Ӽ���ˮ�γ���CH3CH2CH2CH2OCH2CH2CH2CH3����������ˮ��������ϩCH2=CHCH2CH3��ͬʱ�������ӱ�Ũ�����������嵥�ʣ�
�ʴ�Ϊ��ab��
��3������������������ʶԲ�ͬ�����ĺ��������������ԣ����з��ӽṹ�ͻ�ѧ��ɷ���������CH3CH2CH2CH2BrҲ����-CH2CH2CH2CH3�����Բ���ͨ�������������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3����
�ʴ�Ϊ��������������1-�嶡��Ҳ����-CH2CH2CH2CH3��
��4���ᴿ1-�嶡�飬�ռ��������Ϊ1-�嶡�飬�����뽫1-�嶡������������Һ�����������¶ȴ���е㣬
�ʴ�Ϊ��101.6�森
���� ������Ҫ������1-�嶡�����ȡʵ�飬������Ӧԭ���ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | 56gN2��CO�Ļ�������к��е�ԭ������ĿΪ2NA | |
| B�� | ��״���£�11.2L�Ҵ��к��еķ�����ĿΪ0.5NA | |
| C�� | 1L1mol•L-1 MgCl2��Һ�к��е���������ĿΪNA | |
| D�� | 2.7gAl����������Cl2����ȫȼ��ת�Ƶĵ�����ĿΪ0.3NA |
| A�� | 2��1 | B�� | 1��2 | C�� | 1��1 | D�� | 3��1 |
| A�� | Mg+��Al3+��Cl-��NO3- | B�� | Fe2+��Na+��NO3-��SO42- | ||
| C�� | Ba2+��Na+��K+��NO3- | D�� | K+��Ca2+��CO32-��OH- |
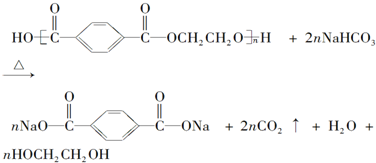
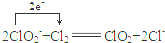 ��
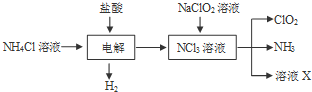
��

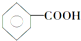 ��CH3CH2CH��CH3��CH3 ��CH4 ��
��CH3CH2CH��CH3��CH3 ��CH4 ��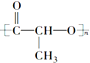 ��
��
 ��
�� ��
��

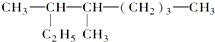 ��������3��4һ�������飮
��������3��4һ�������飮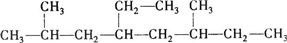 ��1mol������ȫȼ������������18.5mol��
��1mol������ȫȼ������������18.5mol��