��Ŀ����
18����1���л���ı�ʾ�������ֶ����������dz��õ��л���ı�ʾ��������
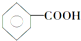 ��CH3CH2CH��CH3��CH3 ��CH4 ��
��CH3CH2CH��CH3��CH3 ��CH4 ��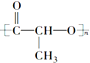 ��
��
��
 ��
�� ��
��
��������ʾ���������ڽṹ��ʽ��Ϊ���٢ڢۢܢߣ����ڱ���ģ�͵�Ϊ���ݣ�
��д�����й����ŵ����ƣ��ǻ���ȩ����
��ں͢�Ϊͬ���칹�壮
IVд���ɵ���ϳ����ʢܵĻ�ѧ����ʽ��

��2����ϵͳ��������д�����л�������ƣ�
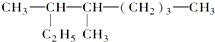 ��������3��4һ�������飮
��������3��4һ�������飮��3��2��6-����-4-�һ�����Ľṹ��ʽ��
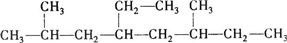 ��1mol������ȫȼ������������18.5mol��
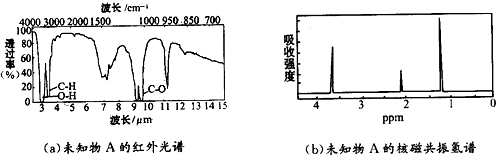
��1mol������ȫȼ������������18.5mol����4��δ֪��A��ʵ��ʽ�ͷ���ʽ����C2H6O��A�ĺ������ͼ��ͼ��a����δ֪��A�ĺ˴Ź���������������ͼ��b���������֮����1��2��3��δ֪��A�Ľṹ��ʽΪCH3CH2OH������Ϊ���Ҵ���
���� ��1���ṹ��ʽ��ָ�ѷ����и�ԭ�����ӷ�ʽ��ʾ������ʽ�ӣ��ṹʽ�DZ�ʾ��Ԫ�ط��źͶ��߱�ʾ������ԭ�ӵ����кͽ�Ϸ�ʽ��ʽ�ӣ�����ģ�;���ԭ�ӽ�������ģ�ֻ�ܷ�ӳԭ�Ӵ�С�����µ����з�ʽ��
-OHΪ�ǻ���-CHOΪȩ����
����ʽ��ͬ���ṹ��ͬ������ͬ���칹�壻���ʢ�Ϊ���۲������࣬2-�ǻ������к����Ȼ����ǻ����������۷�Ӧ���� ��
��
��2����������������ԭ���������ɣ�
��3����������������ԭ��д�����л���Ľṹ��ʽ�����ݸ��л���Ľṹ��ʽ�γɷ���ʽ��Ȼ��������ȫȼ�����ĵ����������ʵ�����
��4��A�ĺ˴Ź���������3���壬˵����������3��Hԭ�ӣ������֮��Ϊ��Ӧ�ĸ���Hԭ�Ӹ���֮�ȣ����ݷ���ʽ��֪������Hԭ������������ȷ������Ŀ���ṹ��ʽ��
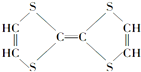
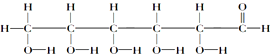
��� �⣺��1��I�����ڽṹ��ʽ���У��٢ڢۢܢߣ����ڽṹʽ���У��ࣻ���ڱ���ģ�͵��У��ݣ��������ģ�͵��У��ޣ��ʴ�Ϊ���٢ڢۢܢߣ��ݣ�
II��-OHΪ�ǻ���-CHOΪȩ�����ʴ�Ϊ���ǻ���ȩ����
III������ʽ��ͬ���ṹ��ͬ������ͬ���칹�壬�ں͢�Ϊͬ���칹�壬�ʴ�Ϊ���ڣ��ޣ�
IV��2-�ǻ������к����Ȼ����ǻ����������۷�Ӧ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��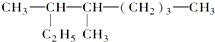 �����л����̼��8��C������Ϊ���飬��Ŵ����·���ʼ�����л�������Ϊ��3��4һ�������飬�ʴ�Ϊ��3��4һ�������飻��3��2��6-����-4-�һ����飬����Ϊ���飬��2��6��C������1��������4��C����1���һ������л���ṹ��ʽΪ��
�����л����̼��8��C������Ϊ���飬��Ŵ����·���ʼ�����л�������Ϊ��3��4һ�������飬�ʴ�Ϊ��3��4һ�������飻��3��2��6-����-4-�һ����飬����Ϊ���飬��2��6��C������1��������4��C����1���һ������л���ṹ��ʽΪ��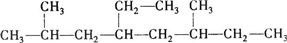 ��������ṹ��ʽ��֪���л������ʽΪ��C12H26��1mol���л�����ȫȼ�����ĵ����������ʵ���Ϊ��12+$\frac{26}{4}$��mol=18.5mol���ʴ�Ϊ��
��������ṹ��ʽ��֪���л������ʽΪ��C12H26��1mol���л�����ȫȼ�����ĵ����������ʵ���Ϊ��12+$\frac{26}{4}$��mol=18.5mol���ʴ�Ϊ�� ��18.5��
��18.5��
��4��������֪�������ַ壬˵����ԭ������������������֮����1��2��3��˵����������ĸ���֮��Ϊ1��2��3������ʽΪ��C2H6O���ʽṹ��ʽΪ��CH3-CH2-OH������Ϊ�Ҵ����ʴ�Ϊ��CH3-CH2-OH���Ҵ���
���� ���⿼�����л����������л���ṹ��ʽ����д��֪ʶ����Ŀ�ѶȲ���ע�����ճ����л��������ԭ��Ҫ��ѧ���ܹ���������ȼ��ͨʽ�����������

| A�� | ��������ˮ�ķ�Ӧ��Na+H2O�TNa++OH-+H2�� | |
| B�� | ������������ȼ�գ�H2+Cl2�T2H++2Cl- | |
| C�� | С�մ�����θ����ࣺH++HCO3-�TH2O+CO2�� | |
| D�� | ���Ȼ�����Һ��ʴͭ������ӡˢ��·�壺Fe3++Cu�TFe2++Cu2+ |
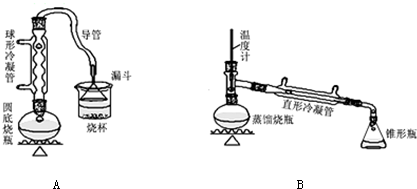
��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���Ǽȿ������ճ�֣��ֿ��Է�ֹ������
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ����ab��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С�-CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���𣺲�����������1-�嶡��Ҳ����-CH2CH2CH2CH3��
��4��Ϊ�˽�һ���ᴿ1-�嶡�飬��С��ͬѧ�������л�����й��������±���
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
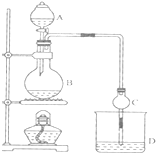 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H50H
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��1��Ũ����������Ǵ�������ˮ�������ú�ͬλ��18O���Ҵ����������ᷴӦ��д���ܱ�ʾ18O λ�õĻ�ѧ����ʽ��CH3COOH+CH3CH218OH$?_{��}^{ŨH_{2}SO_{4}}$CH3CO18OC2H5+H2O��
��2�����θ����C�������Ƿ�ֹ����������������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ���ǣ������ӷ���ʽ��ʾ��CO32-+H2O?HCO3-+OH-����Ӧ������D�е�������Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz��
��3����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ�������Ҵ���Ȼ����������ռ�77�����ҵ���֣��Եýϴ���������������
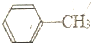 =2KMnO4$\stackrel{��}{��}$
=2KMnO4$\stackrel{��}{��}$ +KOH+2MnO2��+H2O
+KOH+2MnO2��+H2O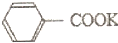 +HCl��
+HCl�� +KCl
+KClʵ�鷽����һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
��֪����������Էַ�������122���۵�122.4�棬��25���95��ʱ�ܽ�ȶȷֱ�Ϊ0��.3g��6.9g�����������л���һ�㶼�й̶��۵㣮
��1������IΪ��Һ������IIΪ����
��2����ɫҺ��A�Ǽױ����������ֱ���A���Լ����������Ը��������Һ����������ɫ��Һ��ɫ��
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ������
���Ʋ���ȷ������ɱ������ݣ�
| ��� | ʵ�鷽�� | �������� | ���� |
| �� | ����ɫ����B����ˮ�У����� �ܽ⣬��ȴ���� | �õ���ɫ�������ɫ��Һ | |
| �� | ȡ������Һ���Թ��У� ���������������ữ��AgNO3��Һ | ���ɰ�ɫ���� | ��Һ��Cl- |
| �� | �����ɫ���壬����ʹ���ۻ��� ����۵� | ����۵�Ϊ122.4�� | ��ɫ�����DZ����� |
| A�� | ��������������ˮ | |
| B�� | �ŵ�Ӱʱ����ӳ���䵽��Ļ�ϵĹ��� | |
| C�� | �Ȼ�����Һ�е�������������Һ���ֺ��ɫ���� | |
| D�� | ����������������γɳ�������ɳ�� |
 ijͬѧ�ú������⣨Fe2O3���ķ���м����ȡ�Ȼ��������װ�ã�ʡ�Լг�װ�ã��������Ѽ�飩��ͼ��ʾ�������ƶϲ��������ǣ�������
ijͬѧ�ú������⣨Fe2O3���ķ���м����ȡ�Ȼ��������װ�ã�ʡ�Լг�װ�ã��������Ѽ�飩��ͼ��ʾ�������ƶϲ��������ǣ�������| A�� | �ձ���H2O2��Һ�����ǽ�Fe2+����ΪFe3+ | |
| B�� | a�е������������ᷴӦ�����Ȼ��� | |
| C�� | b���ռ��������������� | |
| D�� | �ձ�����Һ�����ᾧ�õ��Ȼ������� |
 ��
��